DEVELOPMENT AND VALIDATION OF SIMULTANEOUS HPLC METHOD FOR DETERMINATION OF LIDOCAINE, HYDROCORTISONE ACETATE AND METHYL PARABEN IN ANTI HEMORRHOID OINTMENT
Seyed Majid Sedighi Nalkiashary1*, Mahshid Nikpour Nezhati 2, Homayon Ahmad Panahi 3
|
|
|
Abstract
The present study is aimed to develop a simple, rapid, sensitive and precise high performance liquid chromatographic (HPLC) method for the simultaneous determination of Lidocaine, Hydrocortisone Acetate and Methyl Paraben in Anti Hemorrhoid ointment. This research was carried out by employing an Inert sil ODS C18 column, 5 μ, 25cm × 4.60mm id with a flow rate of 1 ml/min, using a mobile phase combination of Methanol and phosphate buffer (PH 8) in a ratio of 65:35 V/V. Detection was carried out at 230 nm. The retention time of Lidocaine, Hydrocortisone Acetate and Methyl Paraben was found to be 3.93 min, 1.26min, and 7.03 min respectively. The developed method is simple, precise and reproducible. Hence the proposed method can be used for the simultaneous determination of Lidocaine, Hydrocortisone Acetate and Methyl Paraben in Anti Hemorrhoid ointment.
Keywords: Lidocaine, Hydrocortisone Acetate, Methyl Paraben, RP-HPLC, Anti Hemorrhoid ointment, Validation method
Introduction
Introduction
Anti-Hemorrhoid ointment reduces the swelling and relieves the discomfort of hemorrhoids (swellings in the area around the anus) [1]. This consists of Lidocaine, Hydrocortisone Acetate and Methyl Paraben. Structurally, lidocaine is N-(2,6-dimethylphenyl)-N2, N2-diethylglycinamide, lignocaine as shown in fig.1. It possesses anti-scratching, anti-irritation, and anti-stimulation activity. It is known as an anesthetic material to numb tissue in a specific area. Additionally, it is used to treat ventricular tachycardia and to perform nerve blocks [2].
Lidocaine works by stopping the sodium ions from passing through the voltage-gated channels. So the signals for pain are stopped even before the signals formed. Lidocaine binds to the sodium els channels. The amide on the lidocaine allows it to act as amino acid and interacting with it, then it cannot transfer the sodium ions. Lidocaine alters signal conduction in neurons by prolonging the inactivation of the fast voltage-gated Na+ channels in the neuronal cell membrane responsible for action potential propagation [3, 4].
Fig.1. Chemical structure of lidocaine
Hydrocortisone acetate is a synthetic glucocorticoid corticosteroid and a corticosteroid ester. It plays a pivotal role in inflammation reduction by immune suppression [4]. Cortisol is the main glucocorticoid secreted by the adrenal cortex and it is involved in the stress response. Hydrocortisone also is known as 11β,17α,21-trihydroxypregn-4-ene-3,20-dione which is shown in fig.2 [5].
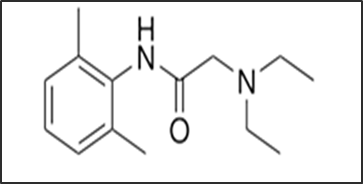
Fig .2. Chemical structure of Hydrocortisone
Methylparaben, Methyl 4-hydroxybenzoate, is the methyl ester of p-hydroxybenzoic acid which is shown in fig.3. It is a preservative and an anti-fungal which is more used in a variety of cosmetics and personal care products [6, 7].
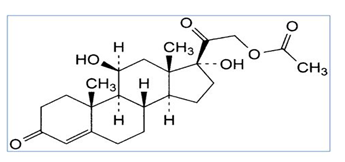
Fig 3. Chemical structure of methylparaben
The purpose of this study was to develop a simple, accurate and precise HPLC method for the determination of Lidocaine, Hydrocortisone Acetate and Methyl Paraben simultaneously in Anti Hemorrhoid ointment and to validate developed method according to ICH guidelines [8-10]. Reversed-Phase high-performance liquid chromatography (RP-HPLC) involves the separation of molecules based on hydrophobicity. The separation depends on the Hydrophobic binding of the solute molecule from the mobile phase to the immobilized hydrophobic ligands attached to the stationary phase, the sorbent [11]. The most commonly employed experimental procedure for the RP-HPLC analysis generally involves the use of a C18-based sorbent and a mobile phase. The chromatographic packing materials that are generally used are based on microparticulate porous silica which allows the use of high linear flow velocities resulting in favorable mass transfer properties and rapid analysis time. The silica is chemically modified by a derivatized silane bearing an n-alkyl hydrophobic ligand-the most commonly used is C18 [12].
Material and Method
Chemicals and reagents
Methanol HPLC grade, reagent potassium phosphate monobasic and sodium hydroxide were purchased from Merck. The double-distilled water, Anti Hemorrhoid ointment and reference standards of Lidocaine, Hydrocortisone and Methylparaben were obtained as a gift from IRAN AVANDFAR pharmaceutical company.
Apparatus
The development and validation of the assay were carried out using HPLC (YOUNGLING YL 9100) system equipped with the pump and UV/VIS detector. The chromatographic analysis was performed using enable C18 G (250 mm× 4.6mm× 5µm) reverse phase column. Clarity software was used as a data processor.
Chromatographic Conditions
1.21 g potassium phosphate monobasic was weighed accurately and solubilized in distilled water (250 ml) and PH was adjusted with sodium hydroxide solution (1 N) to 8 (solution A). The mobile phase of a mixture of methanol and solution A in the ratio of (65:35 %v/v) injected at a flow rate of 1 ml/min with detection at 230 nm. The mobile phase was filtered through a 0.5µm membrane filter and degassed. The operating temperature was set at 30°c and the injection volume was 20 µL.
Preparation of standard solutions
To prepare a standard solution, 27.5 mg of standard Hydrocortisone solution and 20mg of standard Methyl Paraben solution were mixed and were diluted to 50 mL by adding methanol. Then, 1 ml of the resulting solution and 10 mg of standard Lidocaine solution were mixed and diluted to 20 mL by the mixed solution of methanol and water in the ratio of 1:1 v/v.
Preparation of sample solution
Anti Hemorrhoid ointment of about 1 g was weighed and transferred to the orlon. Additionally, it was dissolved in 60 ml methanol. The obtained solution was centrifuged for 30 min to separate different phases of ointment. Then 6 ml of this solution and 4 ml methanol were mixed completely. The obtained solution was filtered through a 0.5 µm membrane filter.
Method validation
The method was validated as ICH guidelines, and the validation parameters included specificity, linearity, accuracy, and precision.
Specificity
To determine the specificity, the analytical method should be compatible to discriminate between the analyte and the other components in the mixture. Also, specificity has been evaluated by injecting 20 µl of placebo and standard solutions.
Linearity
The linearity evaluation of the method was carried out by preparing different standard solutions by diluting the standard stock solutions with the mobile phase in 3 various concentrations (80%- 100% and 120%) of lidocaine, hydrocortisone, and methylparaben standard stock solutions.
Three injections from each concentration were analyzed under the same conditions. Linearity was evaluated using the least square regression analysis. The slope value and correlation coefficient (r2) values were calculated.
Precision and Repeatability
The Precision and repeatability of the proposed method were determined by several measurements of standard solutions and sample solution in 3 concentrations (80%- 100% and 120%). This method was performed three times a day on three consecutive days.
Accuracy
To study the accuracy of the proposed method, three different concentrations (80%- 100% and 120%) of each sample were injected three times. Recovery determined as the percentage difference between the expected and measured drug concentrations. Percentage recovery and RSD were calculated.Results and discussionMethod development and optimization
The purpose of this study was developing and optimizing the reversed HPLC chromatographic method to determine Lidocaine, Hydrocortisone and Methyl Paraben in Anti Hemorrhoid ointment simultaneously. Various factors that were optimized for this method included specificity, linearity, precision (repeatability) and accuracy. The mobile phases consisting of methanol and potassium phosphate monobasic buffer in the ratio of 65:35 v/v with a flow rate of 1 ml/min, injection volume 20 µl and column temperature 30 °c at 230 nm was optimized as the best chromatographic conditions.
Method validation
Specificity
To prove the specificity of the method, the chromatograms of placebo solution and standard solution were compared. For this purpose, 20 µl from solutions were injected into the HPLC system separately and the chromatogram results are shown in figures 4 and 5. It is obvious that there is no interference with the main peak and this confirmed the specificity of the method.
Fig 4. Chromatogram of placebo solution
Fig. 5. Standard chromatogram of lidocaine-Hydrocortysone-methylparaben
Linearity and range
The study of linearity has been shown in table 1 summarily which confirms that acceptable linearity was obtained in 3 concentrations (80%- 100% and 120%). The correlation coefficient (r2) of Lidocaine, Hydrocortisone and Methylparaben was found to be 0.997, 0.997 and 0.999 as given in figures 6, 7 and 8 respectively.
Table 1. Linearity and range of the developed method
|
|
Lidocaine |
Hydrocortisone |
Methylparaben |
|
Concentration rang |
80%-120% |
80%-120% |
80%-120% |
|
Regression equation |
117.8x -1278 |
9.121x -49.80 |
9.190x -167.0 |
|
Correlation coefficient |
r2=0.997 |
r2=0.997 |
r2=0.999 |
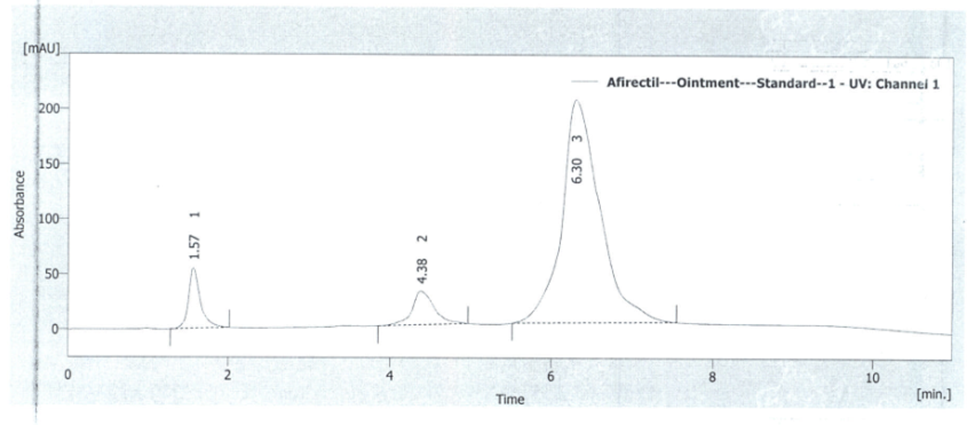
Fig. 6. Linearity curve of lidocaine
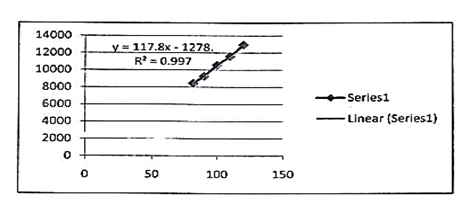
Fig. 7. Linearity curve of Hydrocortisone
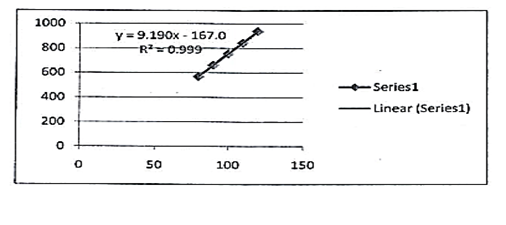
Fig. 8. Linearity curve of methylparaben
Accuracy
The percentage recovery of lidocaine, Hydrocortisone, and Methylparaben have been evaluated at three levels of concentration. The results were within 100±3% which ensures the accuracy of the method. (table 2)
Table 2. percentage recovery
|
Lidocaine - Recovery percentage |
|
|
39.28/40×100=98.20% |
Concentration=80% |
|
49.05/50×100=98.10% |
Concentration=100% |
|
60.14/60×100=100.23% |
Concentration=120% |
|
Hydrocortisone Recovery percentage |
|
|
39.28/40×100=98.20% |
Concentration=80% |
|
49.05/50×100=98.10% |
Concentration=100% |
|
60.14/60×100=100.23% |
Concentration=120% |
|
Methylparaben Recovery percentage |
|
|
39.28/40×100=98.20% |
Concentration=80% |
|
49.05/50×100=98.10% |
Concentration=100% |
|
60.14/60×100=100.23% |
Concentration=120% |
Precision
The precision of the method expresses the closeness of results which are obtained from a series of measurements. Also, the relative standard deviation (RSD) was calculated. %RSD is less than 2% for peak area which ensures precision of the developed method. Retention time of Lidocaine, Hydrocortisone Acetate and Methyl Paraben was found to be 3.93 min, 1.26min and 7.03 min respectively. The results of precision study for 3 consecutive days are summarized in table 3, 4 and 5
Table 3. Determination of RSD in 3 days for lidocaine
|
|
Day3 |
Day2 |
Day1 |
|
|
|
|
|
|
|
preparations |
|
||
|
9148/817 |
9215/692 |
9477/573 |
Injection1 |
Conc.80% |
|
|
|
9191/244 |
9277/425 |
9496/966 |
Injection2 |
|
|
|
|
3Day/Conc.80% |
9209/611 |
9287/254 |
9469/937 |
Injection3 |
|
|
|
9308/303 |
9183/224 |
9260/214 |
9481/472 |
Mean |
|
|
|
154/8302 |
31/1804 |
38/6369 |
13/9426 |
STDEV |
|
|
|
1/66 |
0/33 |
0/41 |
0/14 |
RSD% |
|
|
|
11586/583 |
11521/474 |
11921/828 |
Injection1 |
Conc.100% |
|
|
|
11514/101 |
11564/282 |
11821/437 |
Injection2 |
|
||
|
3Day/Conc.100% |
11595/782 |
11502/695 |
11890/112 |
Injection3 |
|
|
|
11657/59 |
11565/49 |
11529/48 |
11877/79 |
Mean |
|
|
|
191/5498 |
44/74 |
31/5651 |
51/3168 |
STDEV |
|
|
|
1/64 |
0/38 |
0/27 |
0/43 |
RSD% |
|
|
|
12851/824 |
12302/567 |
12634/909 |
Injection1 |
Conc.120% |
||
|
12891/292 |
12249/957 |
12667/58 |
Injection2 |
|
||
|
3Day/Conc.120% |
12859/583 |
12343/466 |
12572/076 |
Injection3 |
|
|
|
12597/03 |
12867/57 |
12298/66 |
12624/86 |
Mean |
|
|
|
285/4742 |
20/91 |
46/8765 |
48/5393 |
STDEV |
|
|
|
2/26 |
0/16 |
0/38 |
0/38 |
RSD% |
|
|
Table 4. determination of RSD in 3 days for hydrocortisone
|
Day3 |
Day2 |
Day1 |
|||
|
preparations |
|||||
|
623/192 |
646/291 |
667/186 |
Injection1 |
Conc.80% |
|
|
624/373 |
622/435 |
650/491 |
Injection2 |
||
|
3Day/Conc.80% |
636/994 |
624/617 |
650/043 |
Injection3 |
|
|
638/402 |
628/186 |
631/114 |
655/907 |
Mean |
|
|
15/23 |
7/6504 |
13/1885 |
9/7707 |
STDEV |
|
|
2/38 |
1/21 |
2/08 |
1/48 |
RSD% |
|
|
818/694 |
835/92 |
796/022 |
Injection1 |
Conc.100% |
|
|
797/992 |
826/459 |
805/657 |
Injection2 |
||
|
3Day/Conc.100% |
798/759 |
798/613 |
800/058 |
Injection3 |
|
|
808/685 |
805/148 |
820/33 |
800/579 |
Mean |
|
|
10/3398 |
11/7371 |
19/3938 |
4/8385 |
STDEV |
|
|
1/27 |
1/45 |
2/36 |
0/6 |
RSD% |
|
|
977/677 |
985/23 |
994/767 |
Injection1 |
Conc.120% |
|
|
976/206 |
976/335 |
985/251 |
Injection2 |
||
|
3Day/Conc.120% |
931/52 |
976/598 |
981/964 |
Injection3 |
|
|
976/1717 |
961/801 |
979/387 |
987/327 |
Mean |
|
|
13/0632 |
26/2344 |
5/0613 |
6/6492 |
STDEV |
|
|
1/33 |
2/72 |
0/51 |
0/67 |
RSD% |
|
Table 5. Determination of RSD in 3 days for methylparaben
|
|
Day3 |
Day2 |
Day1 |
|
||
|
Preparations |
||||||
|
1112/953 |
1099/768 |
1133/229 |
Injection1 |
Conc.80% |
||
|
1136/403 |
1138/322 |
1149/471 |
Injection2 |
|||
|
3Day/Conc.80% |
1142/753 |
1148/384 |
1156/589 |
Injection3 |
||
|
1135/319 |
1130/703 |
1128/825 |
1146/43 |
Mean |
||
|
9/667 8 |
15/6964 |
25/6618 |
11/9732 |
STDEV |
||
|
0/85 |
1/38 |
2/27 |
1/04 |
RSD% |
||
|
1400/623 |
1414/32 |
1489/567 |
Injection1 |
Conc.100% |
||
|
1412/28 |
1408/581 |
1461/903 |
Injection2 |
|||
|
3Day/Conc.100% |
1408/679 |
1384/454 |
1436/239 |
Injection3 |
||
|
1424/072 |
1407/194 |
1402/452 |
1462/57 |
Mean |
||
|
33/4244 |
5/9686 |
15/8483 |
26/6702 |
STDEV |
||
|
2/34 |
0/42 |
1/13 |
1/82 |
RSD% |
||
|
1638/234 |
1664/854 |
1696/329 |
Injection1 |
Conc.120% |
||
|
1673/123 |
1661/944 |
1708/713 |
Injection2 |
|||
|
3Day/Conc.120% |
1611/063 |
1674/255 |
1699/182 |
Injection3 |
||
|
1669/744 |
1640/807 |
1667/018 |
1701/408 |
Mean |
||
|
30/3923 |
31/1098 |
6/4343 |
6/4851 |
STDEV |
||
|
1/82 |
1/89 |
0/38 |
0/38 |
RSD% |
||
Conclusion
In this study, we employed a simple, rapid, sensitive and precise high performance liquid chromatographic (HPLC) method for simultaneous determination of Lidocaine, Hydrocortisone Acetate and Methyl Paraben in Anti Hemorrhoid ointment. For this purpose, different factors such as linearity, specificity, precision, and accuracy have been confirmed by measuring some parameters. The correlation coefficient (r2) of Lidocaine, Hydrocortisone and Methylparaben was found to be 0.997, 0.997 and 0.999 respectively. The accuracy of the method is in the range of 100±3%. %RSD is less than 2% for peak area. The method was validated by ICH guidelines. overall, this proposed method was successful for the determination of Lidocaine, Hydrocortisone Acetate and Methyl Paraben in Anti Hemorrhoid ointment simultaneously.
Acknowledgements
The authors would like to thank the Islamic Azad University and IRAN AVANDFAR pharmaceutical company for equipment and laboratory services.
References
