Preliminary Fractionation and Quantification of Hyoscyamine In the Crude Ethanol Extract of Datura Stramonium Linn Seeds
Idris A.T 1*, Sunday A.M 2, Ibrahim A.I 3, James O.N 4, Salisu A5
|
|
|
ABSTRACT
Introduction: Datura stramonium Linn (D. stramonium L.) is an annual plant that belongs to the Solanaceae, a plant family that is poisonous and emits an unpleasant odor due to the presence of tropane alkaloids. Aim: The study was designed to determine the amount of hyoscyamine in the crude ethanol extract of Datura stramonium L. seeds. Materials and Methods: Fresh D. stramonium L. seeds were procured, identified, extracted, and fractioned in chloroform. The percentage yield was determined. The presence of alkaloids was qualitatively confirmed by phytochemical screening and thin-layer chromatography. The retention factor was determined. The high-performance liquid chromatography (HPLC) of the samples was carried out and the calibration curve was plotted to determine the amount of the hyoscyamine fraction. Results: An 11.04 µg/ml amount of hyoscyamine was obtained at the limit of detection (LOD) and quantification (LOQ) of 0.75 and 2.26 µg/ml respectively. Conclusion: In conclusion, the fraction of hyoscyamine in the D. stramonium L. seeds was little compared to the quantity of the crude seeds.
Key Messages: The extraction and fractionation of hyoscyamine from the crude ethanol extract of Datura stramonium Linn seeds reveal the presence of several phytoconstituents including alkaloids. The hyoscyamine yield was little when compared to the crude sample of the seed used.
Keywords: Chromatography, fraction, hyoscyamine, seeds, rf-value
Introduction
Datura stramonium (D. stramonium) is an annual plant that belongs to the Solanaceae, a plant family that emits an unpleasant smell due to the presence of tropane alkaloids [1]. All parts of the plant are toxic, especially the ripe seeds which contain the highest concentration of the active compound [2]. It contains several alkaloids, such as atropine, scopolamine [3, 4], and hyoscyamine. Other phytoconstituents include; megastigmane, sesquiterpenes, tannins, phlobatannins, cardiac glycosides, carbohydrates, and flavonoids, which are present primarily in the seeds and flowers [5]. The phytoconstituents vary with the species, developmental stage, environment, and part of the plant. The seeds are believed to be responsible for the anticholinergic activity of the plant [6]. Datura intoxication is characterized by anticholinergic symptoms [7] in both the central and peripheral nervous systems. The herb plays a significant role traditionally in the disease treatment due to the mind-altering property [8] it possesses. The fruit juice is used for the treatment of dandruff and falling hair when applied to the scalp [9]. It is applied to soothe painful wounds and sores [10]. Hyoscyamine is one of the predominant alkaloids of D. stramonium [10] that is used as anti-peristaltic/spasmodic [11] in the treatment of gastrointestinal-related disorders. The seeds of this herb are used to calm hysteric, psychotic, and insomnia patients [12]. It also uses to relax smooth muscles in the bronchial tube during asthmatic bronchial spasm [13].
In Nigeria, D. stramonium L. seeds are consumed in a mixture of a locally made juice, Zobo by the youths especially at festivities to induce hallucinogenic sensation. This results in several cases of poisoning with fatal consequences. The fact that the plant constituents vary with location, soil region, pH, and time of harvest, calls for an evaluation of its poisonous phytoconstituents such as hyoscyamine. However, a few pieces of literature are available on the amount of hyoscyamine content in the crude extract of D. stramonium L. The present study was designed to quantify the amount of hyoscyamine in the crude ethanol extract of D. stramonium L. seeds. Plants are considered as a source of novel pharmaceutical products and inexpensive raw material for the synthesis of some known drugs, as they have thousands of active secondary metabolites [14-17].
Aims: to determine the amount of hyoscyamine in the crude ethanol extract of Datura stramonium Linn seeds
Settings and Design: study was quantitative carried out at the Human Anatomy Department, Faculty of Basic Medical Sciences, College of Medical Sciences, Ahmadu Bello University Zaria, Kaduna State, Nigeria.
Methods and Material
Ethical approval with registration ABUCAUC/2018/042 was obtained from the Ahmadu Bello University Committee on Animal Use and Care.
Plant material
Fresh Datura stramonium seeds were procured from Sharada residential area of Nassarawa Local Government, Kano State, Nigeria. The seeds were identified and the voucher number (VN108) was issued at the herbarium of Botany Department, Faculty of Life Sciences, Ahmadu Bello University, Zaria, Kaduna state, Nigeria. The seeds were separated from the pods, washed thoroughly with clean tap water, and air-dried under shade. Two thousand grams of the dried seeds were weighed using a digital weighing machine (LB-1000, China), grounded to a pulp using an electronic blender. The powdered sample was collected into a sterile cellophane bag and kept in a cool dry place for extraction.

Figure 1: D. stramonium L. (A): plant, (B): ripe fruits, (C): seeds, (D): powder
Extraction of D. stramonium L. Seeds
Extraction was carried out using cold maceration according to Djilani et al. [18]. The 200g of pulverized seeds was extracted in 1, 500 ml of 70% (v/v) ethanol at room temperature for 72 hrs (figure 2a). The extract was filtered and the solvent was evaporated in a water-bath at 40 °C (figure 2b). The residue, dissolved in H2O and acidified with H2SO4 to pH 3-4, was extracted with petroleum ether and diethyl ether to remove lipophilic, acidic, and neutral material. After basifying the aqueous solution to pH 9-10 with NH4OH (25%, m/m), it was extracted with chloroform. The extract washed with distilled water to neutral pH, dried with Na2SO4, and concentrated to dryness under reduced pressure to obtain crude alkaloids. Preliminary phytochemical analysis was carried out and the percentage yield of the crude extract was determined as;
% Yield = Weight of the extractInitial weight of the sample × 100
× 100
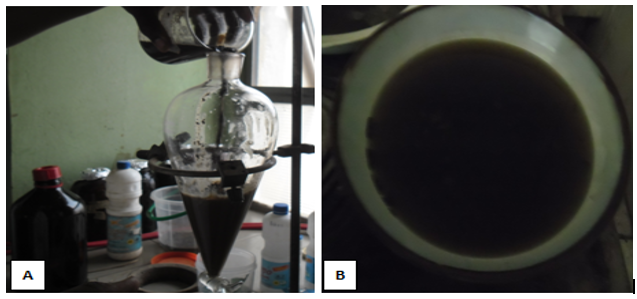
Figure 2: Ethanol extraction of crude D. stramonium L. seeds (A): cold maceration, (B): Ethanol extract on water-bath.
Fractionation of Hyoscyamine
The fractionation was carried out according to Salamah and Ningsih [19]. Five grams of the viscous extract was dissolved in 10 ml of water (figure 3a). Then, the solution was poured into a separating funnel, 10 ml of chloroform was added, and shaken to solve with two phases, namely water, and chloroform. These two phases were separated and collected (figure 3b). This was repeated until the chloroform phase had the same color as the chloroform solvent (figure 3d). The chloroform was then evaporated and recrystallized to obtain the hyoscyamine fraction (figure 3e). The alkaloid was analyzed with UV-Vis spectrophotometric method. The extraction and fractionation were carried out at the Department of Pharmacognosy, Faculty of Pharmaceutical Sciences, Ahmadu Bello University Zaria.
% Yield = Weight of the fractionInitial weight of the sample × 100
× 100

Figure 3: Fractionation of hyoscyamine fraction of D. stramonium L. seeds
A: Crude ethanol extract of the seeds dissolved in hot distilled water, B: Basification, partitioning, and dissolution of the crude extract of the seeds with ammonia, hydrochloric acid, and chloroform, C: Evaporation the of fraction in water-bath, D: Re-purification of the acid layer with ammonia, E: Evaporation of the chloroform layer, F: Hyoscyamine fraction of D. stramonium L. seeds in Eppendorf’s tube.
The thin-layer chromatography (TLC)
With a pencil, a thin mark was made at the bottom of the pre-coated silica plate about 0.5 cm from the bottom to serve as the origin of the applied sample spots. Then, samples solutions (standard l-hyoscyamine powder and fractionate aliquot) were then applied separately and parallel to each other on the spots marked at the baseline at equal distances respectively. The mobile phase Butanol – Acetic acid – Water (BAW) in ratios of 4:1:1 was poured into the TLC chamber to a level of few centimeters above the chamber bottom. For the side of the plate with the sample line to face the mobile phase, the plate prepared with the sample spot was placed in the TLC chamber. The chamber was closed with a lid. This was allowed for spots to sufficiently develop to the solvent front of about 1 cm before the plate apex. After 1 hour, the plate was removed, sprayed Dragendorff’s reagent, and heated in an oven for 5 minutes. The plate was visualized in a visible light chamber for the presence of the spots and calculation of Rf value [20].
Rf = 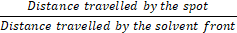
Where: Rf = Retention factor.
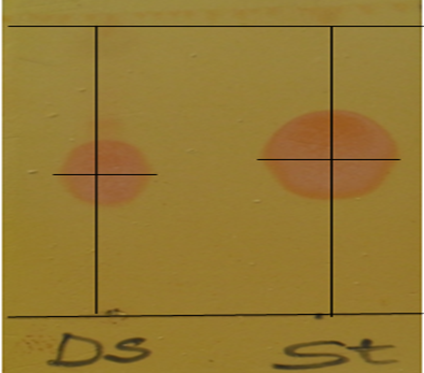
Figure 4: Sprayed chromatographic plate showing orange-colored spots of hyoscyamine in the standard powder (St) and the D. stramonium L. sample (Ds)
The high-performance liquid chromatography quantification of the hyoscyamine fraction
A reversed-phase Techsphere 50DS C18 HPLC column (25 cm × 4.6 mm i.d.) particle size 5 µm, Supelco, Bellefonte, PA, USA) with oven temperature, 40°C in conjunction with a UV adsorption detector operating at 230 nm was employed. The mobile phase was a mixture of 20% acetonitrile, and 45 % methanol, 35 % water (H2O), and 0.1 mol/L phosphoric acid which adjusted the pH to 7.0 and was used at a flow rate of 1 ml/min. A 1-mg aliquot of standard l-hyoscyamine (Sigma, St. Louis, MO, USA) was weighed using an electronic chemical balance (Shimadzu, USA) and was dissolved in 10 ml of standard methanol to make a stock solution of 100 mg/µL. A 6.4 ml of the stock solution was added to 10 ml of the methanol to make a 64 mg/µL solution. Two more solutions of concentration, 32 and 16 mg/µL respectively of the standard hyoscyamine were prepared by serial dilution to allow for a total of three graded solutions for the quantifying the hyoscyamine concentration amount in the sample fraction.
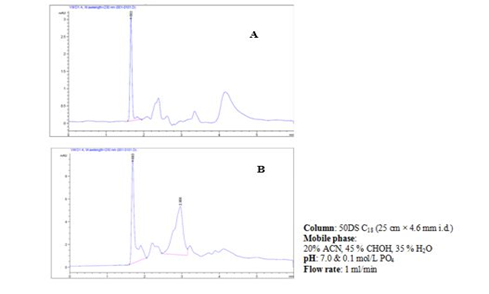
Figure 5: a&b, HPLC chromatogram of Ɩ-hyoscyamine standard powder (A) and hyoscyamine fraction of D. stramonium L. seeds (B).
The determination of the standard curve
The calibration curve for hyoscyamine was constructed by plotting the peak area of samples versus their concentrations (figure 6). The equation of a straight line was established, where x represents the concentration [µg/ml], while y = the area of the peak from the sample. The quantity of hyoscyamine compound in the D. stramonium L. ethanol extract was calculated at the moment that the area of the sample peak was reached using the equation. The area of the peak for hyoscyamine was taken from the chromatogram of D. stramonium L. fraction. The Department of Pharmaceutical Chemistry, Faculty of Pharmaceutical Sciences, Ahmadu Bello University Zaria provided the location for all the analyses that were carried out.
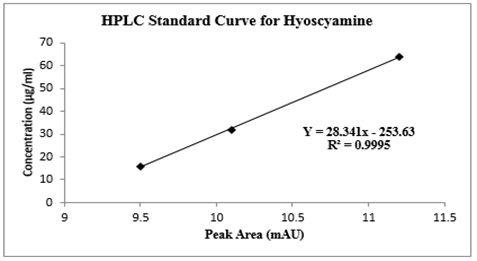
Figure 6: Standard curve for the quantification of hyoscyamine fraction using High-Performance Liquid Chromatography (HPLC) Analysis
Statistical analysis used: The percentage yields were expressed as the simple fraction of the samples and total crude samples. LINEST function test was used to develop the regression equation using the Microsoft Excel program.
Results and Discussion
Hyoscyamine and scopolamine are the major tropane alkaloids aside from several minor alkaloids identified in Datura species. Some of the minor alkaloids in D. stramonium include; tigloidin, aposcopolamine, apoatropin, [21-24]. Table 1, shows phytoconstituents present in the crude ethanol extract of d. stramonium L. seeds. The present phytochemical analysis of the crude D. stramonium L. seeds showed that alkaloids, carbohydrates, saponins, cardiac glycosides, steroids, triterpenes, flavonoids, and tannins were all detected. This was in keeping with [2, 25, 26], which revealed the presence of similar phytoconstituents present in higher concentrations [27].
Table 1: Phytoconstituents Present in the Crude Ethanol Extract of D. stramonium L. Seeds
|
Result |
Confirmation |
|
|
Alkaloids |
|
|
|
A reddish-brown precipitate was formed |
+ |
|
A reddish-brown precipitate was formed |
+ |
|
A whitish creamy precipitate was formed |
+ |
|
|
|
+ |
|
Carbohydrates |
|
+ |
|
A violet ring was formed |
+ |
|
A brick-red precipitate was formed |
+ |
|
|
|
+ |
|
Saponins |
|
+ |
|
A thin layer foam was formed |
+ |
|
|
|
+ |
|
Cardiac glycosides |
|
+ |
|
A blue coloration was formed |
+ |
|
|
|
+ |
|
Tannins |
|
+ |
|
A dark green coloration was formed |
+ |
|
|
|
+ |
|
Flavonoids |
|
+ |
|
A yellow fluorescence was formed |
+ |
|
|
|
+ |
|
Steroids & Triterpenes |
|
+ |
|
A green coloration was formed |
+ |
In D. stramonium L. seeds, hyoscyamine, and scopolamine account for 66% and 20% of the total tropane alkaloid content, respectively [28]. Our study observed that the percentage yield of crude ethanol extract of the seeds was 8.04%, while that of the hyoscyamine fraction from the crude extract was 3.60%. This suggested a relatively low yield of the fraction compared to the total crude extract used. The relatively low yield observed could be explained by the fact that hyoscyamine, which exists in a racemic mixture (1:1) of (-) -hyoscyamine and (+)-hyoscyamine, might have undergone some enantiomerisation thereby converting of (-)-hyoscyamine to (+)-hyoscyamine thus enabling the two enantiomers of hyoscyamine to coexist in a sample [29], thereby reducing the percentage abundance of the (-) -hyoscyamine. The enantiomerisation of hyoscyamine from (-) to (+)-hyoscyamine is possible under aqueous alkaline solution (pH > 9) combined with elevated temperatures (> 80 °C) conditions. Although cold maceration during crude ethanol extraction, the fractionation process involved heat application and basification of the sample at different stages. This has altered the optimal acid-base equilibrium in favor of the enantiomerisation. 63-70% of hyoscyamine and 42-80% of scopolamine were the decreases reported. Although part of the plant from which the extraction was made differed, the variation in the yield might also result due to intraspecies variation in genetic makeup, stage of development, duration of plant collection, and the difference in geographical location.
Figure 4 shows two orange spots of the hyoscyamine in the extract and the standard powder was qualitatively identified using thin-layer chromatography. The chromatogram of the standard powder shows an enlarged orange spot of Ɩ–hyoscyamine sulphate (St) on the right-hand side, compared to a relatively small spot (Ds) of the extract on the right. Comparatively, the chromatogram of the sample resembled each other with regard to the position, size, and color. This proved that the hyoscyamine was the compound in the sample. The retention factor (Rf) value determined were 0.53 and 0.48 for the standard powder and the sample hyoscyamine fraction respectively, which suggested that both the sample and standard powder were alkaloids. This has confirmed the presence of alkaloid while the similar rf-value has confirmed alkaloid was hyoscyamine. Orange coloration was a typical alkaloid qualitative chromatographic test. In a similar study [30], orange coloration in the chromatogram plate of the tropane atropine and scopolamine alkaloids for the standard atropine sulfate and scopolamine hydrobromide with position and size that resembled the chromatogram of their standard powders observed.
Table 2. shows the graded doses of standard Ɩ-hyoscyamine powder, peak areas, and retention time produced by the HPLC for the quantification of hyoscyamine in the D. stramonium L. seed. The peak areas were dose-dependent. One calibration curve was obtained for hyoscyamine representing the peak area dependent on concentration based on the areas obtained. Figure 5a shows Ɩ-hyoscyamine (above) and figure 5b hyoscyamine fraction (below) chromatograms respectively. In both cases, the retention time was 1.660 and 1.683 min respectively. A good correlation of linearity has been achieved (n = 3; R2 = 0.9995) and, in the range of 1–10 µg/ml for hyoscyamine. A limit of detection (LOD) and limit of quantification (LOQ) of the fraction was also found as 0.75 and 2.26 µg/ml respectively. Based on the HPLC chromatogram, the hyoscyamine came out from the sample at 1.683 minutes. In table 4, the quantity of the hyoscyamine in the seeds was 11.04 µg/ml obtained from the standard curve using 8250 µg/ml of the hyoscyamine fraction at peak area was 55.31 mUA*S. In a similar study [26], the alkaloids quantity in the ethanol extract of D. stramonium L. seeds was reported as 1.7 mg/mL, out of which 1.13 mg/mL for atropine and 0.57 mg/mL for scopolamine. High-Performance Liquid Chromatography (HPLC) was reported to be one of the most commonly used techniques in alkaloids analysis [31]. We observed a relatively small quantity of the hyoscyamine, the lower limit of detection and quantification in the sample fraction. This was not a surprise as commercial production of alkaloids was generally considered to yield low, especially for hyoscyamine [32]. A study that quantified hyoscyamines in Datura innoxia and found a relatively even lower amount of the compound [33]. It was generally however reported that the main alkaloid in seeds of D. stramonium L. was the atropine although scopolamine, an enantiomer of hyoscyamine also present in significant amounts [34, 35], and that transformation of hyoscyamine-scopolamine occurs in Datura species. This probably could have attributed to the lower yield of the hyoscyamine in the fractioned sample.
Table 2: The standard quantity of hyoscyamine samples and areas
|
Quantity (µg/ml) |
Peak Areas (mUA*S) |
Time/min |
|
16 |
09.4576 |
1.664 |
|
32 |
10.1167 |
1.667 |
|
64 |
11.1797 |
1.660 |
Table 3. shows the quantity of the hyoscyamine fraction of the ethanol extract of D. stramonium L. The absorption peak for hyoscyamine was at 1.683 minutes. Comparing the result with the absorption spectrums of standards hyoscyamine, this shows that the sample was correctly identified. Using the equations from the standard curves of standards solutions and that of the peak area from the extract, the alkaloids quantity in µL of the sample was calculated. Based on this, the concentration of the hyoscyamine alkaloid was calculated in the fraction of the D. stramonium L. seeds.
Table 3: Standard Quantity of Hyoscyamine Samples and Areas
|
Quantity (µg/ml) |
Peak Areas (mUA*S) |
Time/min |
|
16 |
09.4576 |
1.664 |
|
32 |
10.1167 |
1.667 |
|
64 |
11.1797 |
1.660 |
Table 4: Hyoscyamine Quantity In the Fraction
|
Conc. (µg/ml) |
Peak Areas (mUA*S) |
Time/min |
Qty (µg/ml) |
|
8250 |
55.31 |
1.68 |
11.04 |
Conclusions
D. stramonium L. seeds contain several phytoconstituents including alkaloids. Although the fraction hyoscyamine was little when compared to the crude seed extract, this may suggest the high toxicity potential of the compound.
Acknowledgment
We sincerely appreciate Mallam Kabiru, Mallam Saidu Bala-riti, Abubakar Chawai Sulaiman of the Departments of Pharmacognosy, Pharmacology, and Pharmaceutical Chemistry for the assistance rendered in the course of this research.
References
Appendix-1

