Toxic Effect of Haloperidol on The Kidney of Chick Embryos
Fawzyah Abdullah Mohammed Al-Ghamdi
|
|
Department of Zoology (Embryology)-Faculty of Science-Jeddah University, Jeddah, Saudi Arabia. |
ABSTRACT
The kidney is a vital organ in the body and is severely affected by toxicant and poisonous substances and is considered the first organ that demonstrates the symptoms of drug toxicity. The current work aimed to investigate the impact of haloperidol treatment (0.25 mg/egg) on the renal development of chick embryos. The haloperidol was given at different developmental stages, at (5-day), the first week of incubation, and at (11-day) the first week of incubation, to study the teratogenic effect on the development of the kidney. Also, the morphological and histological studies were performed on the liver of 7-, 10-, 14-, and 16-day-old chick embryos treated with (0.25 mg/egg) of haloperidol at (zero, 5th, and 11th days) of incubation. Treatment with Haloperidol drug-induced retardation in the growth of the developing kidney of the treated chick embryos. The sign of retardation was observed in the treated kidney of chick embryos. The group that was injected on day zero was seriously influenced, followed by those injected on the 5-day incubation. The toxic effects of the drug were also observed more at 16-day treated chick embryos. It is concluded that that haloperidol has dangerous effects on kidney development with powerful toxicity on the cells, and the effect induced by the drug depends on the time of administration.
Keywords: kidney; antidepressant drug; haloperidol; degeneration; chick embryo.
Introduction
Pregnancy in women is considered one of the crucial stages in the life of a woman and one of the most stressful factors affecting the females during gestation [1, 2]. Therefore, women are suffering from depression overmuch predominately than men particularly during gestational stages, and frequently cluster in the childbearing years [3, 4].
Many investigators found that the treatment of severe depression during pregnancy and lactation is a particularly risky condition and has not yet been safe in the use of three-ring antidepressant drugs (TCAs) [5-7].
Some studies reported that the administration of antidepressant drugs during pregnancy has not yet been authorized due to the reports of fetal anomalies in the nervous system and delayed in fetal growth [8, 9]. Moreover, some authors stated that depression in women is more common than men with a rate of (2:1) and depression in women is different depending on the period of their lives [10, 11]. Generally, most therapies that are used for treating depression have neurological and anti-dopamine effects, leading to changes in endocrine secretions [12, 13]. Also, some researchers recorded that anti-neurological drugs lead to the deficiency of estrogen and progesterone, in addition to inhibition in the secretion of growth hormone [14, 15].
Baldessarini and Tarazi [16] found that (TCAs) drugs possessing a diuretic effect as a result of these medications and can lead to an alteration in the filtration rate due to a reduction in the blood pressure.
Several studies dealing with (TCAs) drugs revealed that these therapies are highly associated and the protein of the cellular membrane. Their metabolites are collected in the brain, lung, and other tissues, as well as enter the fetal blood circulation and breast milk, and cannot be eliminated through dialysis [17-21].
Other researchers reported that haloperidol is one of the most commonly used kinds of an antidepressant drug [22-24], where some documents revealed that haloperidol can lead to delayed growth in the body, which was one of the most important toxic symptoms of this drug [25-27]. Also, some studies showed another adverse action of haloperidol drug represented in inducing degenerative changes in the nervous system, and therefore haloperidol is considered as a neurodegenerative drug [28, 29].
In a study carried by De Vries et al. [30] they found that a 40% decrease in the mRNA of brain cells of embryos treated with 2 mg/kg on the 14th day before birth. The toxic effect of haloperidol drug on the nervous system is explained by some investigators [31, 32] and they showed that the neurotoxicity of haloperidol may be initiated with the cationic metabolites of haloperidol, HPP+ and RHPP+ (products of haloperidol decomposition) formed by oxidation and reduction pathways. Moreover, Zhuravliova et al. [33] also reported that haloperidol causes neurotoxicity by interacting with NMDA receptors where it causes inhibition of protein-membrane formation and change of cellular membrane organizations, leading to cell death.
Some authors demonstrated that the exposure of the expectant mother to haloperidol in the period of fetal inactivity and the pregnancy leads to a decrease in the weight of all parts of the brain and body [34].
Several authors [35-38] reported in their studies that the kidney goes through three successive stages of growth: Pronephros, mesonephros, and Metanephros. Otherwise, some researchers reported that the middle kidney starts to work from the 5th day and reaches the highest level of function on the 11th-day, and then shows its manifestations of decomposition and gradually decreases its function even before hatching [36, 38]. Also, the general anatomy of the kidney reveals that the metanephros is made up of several lobes, each two-part cortical and medullary pulp. The cortical part contains the upper and lower tubes while the pulp contains an (HL) and the (CT) and the (U) [39-41]. Moreover, Megahed [39] described some decomposition changes in the (MS) starting from (9 to 16 days). It was possible to distinguish the (MT) on the 7th day. At the age of 16-20 days. Whereas, in human beings, there are three types of renal formation in human embryos: the Pronephros and these disappear completely and have no function, the second is the Mesonephros and has a function for a period Short and then comes the metanephros [40, 41].
Material and Methods
The fertilized eggs of chicken were used for its ease of manipulation and administering the drug by injection inside the air chamber [42] And because it gives the same distorting effect of drugs in mammalian embryos. [43, 44] Haloperidol was injected in the air chamber at a dose equal to the normal human therapeutic dose [equivalent to human therapeutic dose] (25-250mg) [16]. The therapeutic dose per egg is calculated which is equal to 0.25 mg/egg. This dose is then diluted with pure natural sesame oil. In this study, the number of (60) eggs was used and divided into two main groups: -1: control group (20) eggs -2: treatment group (40) eggs, with dose (0.25 mg/egg) of (haloperidol). The groups under this group are divided into two classes (T0, T1 classes):(T0) was injected on the 0-day before incubation. (T1) was injected in the 5-day incubation and collected embryos for all groups at ages (7, 10, 14and 16).
Results and Discussion
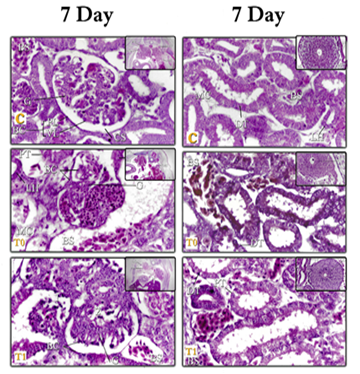
Figure 1: kidneys tissue of chicken embryos at age 7 days, E & H X400 – C= Control; T0=Treated group T0; T1+Treated group T1. Right (Mesonephros (MS) of 7day) – left (Mesonephros 7day)
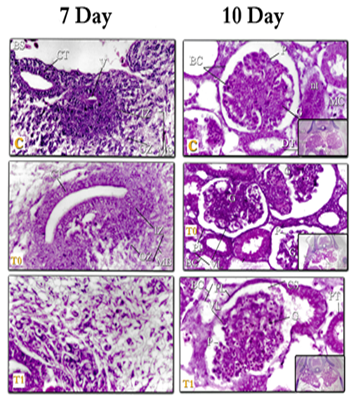
Figure 2: kidneys tissue of chicken embryos at age 7& 10 days, E & H X400 – C= Control; T0=Treated group T0; T1=Treated group T1. Right (Metanephros 7day) – left (Mesonephros 10day
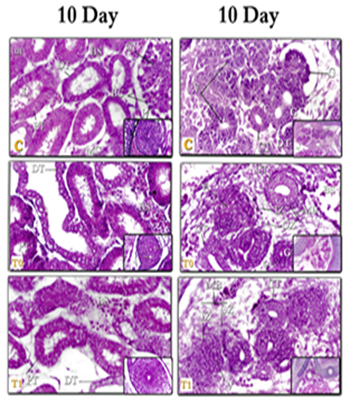
Figure 3: kidneys tissue of chicken embryos at age 10 days, E & H X400 – C= Control; T0=Treated group T0; T1=Treated group T1. Right (Mesonephros 10day) – left (Metanephros 10day)
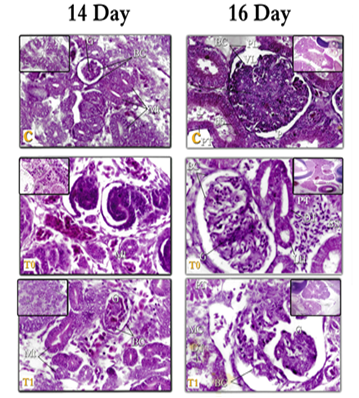
Figure 4: kidneys tissue of chicken embryos at age 14 days, E & H X400 – C= Control; T0=Treated group T0; T1=Treated group T1. Right (Mesonephros 14day) – left (Mesonephros 14day)
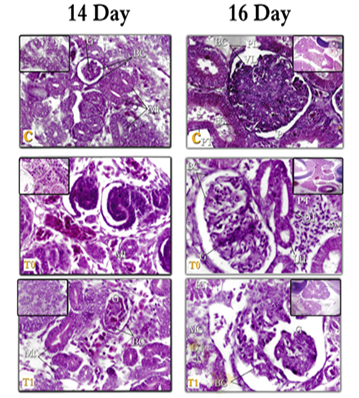
Figure 5: kidneys tissue of chicken embryos at age 14&16 days, E & H X400 – C= Control; T0=Treated group T0; T1=Treated group T1. Right (Metanephros 14day) – left (Mesonephros 16day)
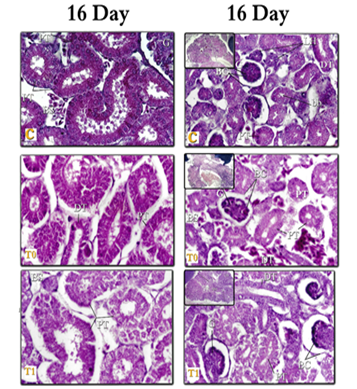
Figure 6: kidneys tissue of chicken embryos at age 16 days, E & H X400 – C= Control; T0=Treated group T0; T1=Treated group T1. Right (Mesonephros 16day) – left (Metanephros 16day)
a) Normal growth of the kidneys in chicken embryos (controlled sample):
At the 7-day the mesonephros (MS) appears as a pair of organs extended inside the body cavity Behind the peritoneal membrane on either side of the track and sideways to the gonads. As growth progresses, the (MS) becomes abdominal for the metanephros (MT) and sideways for the glomerular (G). These results are consistent with those of many researchers who studied the early growth of the (MS). Many investigators [36-38] reported that the (MS) in the embryo of the chick appears as two organs extending within the body cavity on both sides of the tract and central to the middle and abdominal canal of the (MS), and the abdomen of the middle part of the main dorsal vein (Fig. 1).
Whereas in human beings, many studies also confirmed that the (MS) in humans starts from the upper pectoral piece to the third lumbar vertebrae, then the (MS) becomes two oval organs On both sides of the line of the middle body [44-49].
-The renal tissue is formed from vesicles (V) and mesenchymal cells (MC), to form a cup-shaped structure. At 7-days, the middle-shaped kidney tubes increase in number and length and form S-shaped tubes, where are differentiated into (PT) and (DT) and (LH), and the (MD) is located sideways back to the (MS).
These findings are similar to the findings of previous studies [35-37] they found that the tubes of (MS) include (PT), and (DT). The (MD) is lined with vertical epithelial cells elliptical nucleus and its cavity is large. Also, the (MS) tubules in man with different durations are described by some authors [50, 51]. Regarding the amphibians and upper vertebrates, Balinsky and Fabian [52] found that the (MS) tubes in most amphibians and all the upper vertebrates grow from renal tissue through secondary clusters of mesodermal cells. Also, Wettstein and Tiedman [53] recorded that the kidney nephrons at the age of 18-day in the rabbit embryo are made up of RC, and (PT) and (DT) with the absence of (LH) and medulla. However, Megahed [39] confirmed that at the age of 8 days the tubes increase the shape of the In the number and length and the shape of the Letter S, and with the age of 8-15 days, the tubes differentiate to PT, and DT.
-The (RC) of the (MS) form of a cup-shaped structure with a glomerular (G) and Bowman capsule (BC), a glomerular (G) made up of several tufts filled with red blood cells. The (BC) is mounted from a proximal tubule (PL) and vesicular layer (VL). Between these two layers, there is a capsule space (CS). The (RC) then increase in number and become more developed and more differentiated.
These results are similar to the findings of several investigators [35-37, 39, 48, 54] that were collected that the (RC) It's made of the (G) and the (BC). The (G) is made up of large rolls of tuft with connective tissue, the (G) consists of an inner epithelial layer with foot cells and an outer epithelial layer separated by an irregular (BS).
- The beginning of the occurrence of decomposition in the (MS) at the 10-days of incubation and then the (MS) decreases in size gradually. The (MS) appears before the (MT) because it is a major exorcism during early fetal life, while the (MT) is delayed in appearing and perhaps this is actually because its function is in the late embryonic life when the (MS) declines in growth, and then continues (MT) function in puberty. These results are agreed with Leeson [55] who said that the (MS) at the age of 19 days in the rabbit embryo shows future decompositions in the most vertical glomerulus, this decomposition continues in the tail direction Up to the age of 22 days. Several authors agreed that the (MS) in humans reaches its maximum decomposition during the second month of pregnancy, and at the beginning of the third month, most of the (MS) tubes and (G) disappear [49-51, 56].
Previously, Mihalkovics [57] found that the onset of decomposition in the (MS) occurs on days (8-9 days) of incubation, whereas, many other works [31, 36, 39] mentioned that the (MS) is gradually reducing the size of the kidneys and appearing made up of residues of decaying (RC) with an increased fiber ratio among its components. and the (MS) loses its function at the time of hatching.
-The (MT) are divided transversely into three lobes. Each lobe is divided into many lobes separated from each other by a barrier of thin connective tissue. There's also a (CV) and a (U) in the kidney tissue. Each lobe is a Pear-shaped and divided into a two-part structure: the medullary region includes the (LH) and the (CT). And the cortical region includes (RC), (PT), and (DT). And each lobe is emptied into the (CT) which that unites each other to be the (U). These results are similar to Leeson [55] who said that medullary in the (MT) of rabbit includes two types of tubes (HT) and (CT). Concerning the chickens, several researchers [37, 39, 58, 59] described that the (MT) of the chicken bird is seen on 12- day and appears divided into three hardly defined sections (front, middle, and back) and each section consisting of multiple lobes, each of which is emptied into one branch of a ureter (U).
-The current work revealed that the Metanephros (MT) is located back to the Mesonephros (MS), the (MT) consists of many tubes surrounded by dense cells called the non-formed primitive metanephrogenic blastema (MB) of the (MT) and consists of two types of cells: internal region (IZ) and external region (OZ). The whole tubes are lined with vertical epithelial cells with a small empty cavity.
The results of this point are consistent with: many investigators in chick embryo [36, 37, 39] and inhuman; [9, 50, 51, 56] who described that the (MT) is produced from two distinct embryonic foundations: 1 - the renal tissue that supplies the material to the outside, which is the (RC) and the developing detachment tubes. 2) The back of the (MD), which grows by branches within the (CT) and the specified ureter.
-In the present study, at the age of 7-14 days, small clusters of small circular cells appear within the (IZ) from the primitive foundation of the (MB) around the whole tubes. Then a small cavity arises within the clusters of cells converted into vesicles (V), then increases the vesicles in the number and length of the tube formed in the form of the letter S one end of each tube tanged to be cup-shaped called (RC). These findings are consistent with the results of previous studies [37, 41, 60] in Chick (7 and 8 days), Human (week 7) and dog (2-4 days) respectively " that the tissues of the inner region from the primitive foundation of the (MT) begin to arrange itself in the form of micro-cells and later small cavity arises within the cells (Fig. 4).
Concerning other species of animals, many works [39, 61, 62] studied the (MT) in the embryo of the mouse (14-16 days) and the pig respectively, and said that the formation of renal tubes to two stages: 1) - renal vesicles. 2) – s-shaped convoluted objects which are later (CT) and (G) (Fig. 5).
-The (RC) appear in the (MT) first in the form of a cup-shaped structure. Each (RC) is a component from a (G) and a (BC). The latter includes two layers: the outer (PL) and the (VL), and between these two layers, there is a (BC). The (G) is formed from capillaries and connective tissue cells, the (MT), increases (RC) in number and becomes good growth, especially in the deeper part of the cortex.
These results are similar to Leeson [55] who found that (RC) in the rabbit embryo is 15 days old showing different signs of their degree of growth, the (RC) are positioned by the cortex and at the early stage of differentiation.
Several previous studies [33, 44, 58]; revealed that in the kidney of an adult human, all (RC) are made up of: (G) and (BC). The (G) is made up of strands of capillaries wrapped in blood vessels with a small connective tissue. The cells are squamous in the outer wall; specialized in the visceral wall (foot cells), and between the (G) and (BC) there is a (CS).
- In the present work, the (MT) tubes in 14 days of incubation, are numerous and become more mature and can be distinguished to Tubes PR and DT, and from the age of (16 days) of incubation, these tubes are good-growing and can be distinguished to (PT), (HL), (DT), dense spot and (CT). These results are consistent with: Leeson [55] in rabbit embryos, Sadler [44] & Hamilton and Mossman [51] in humans, Sikri and Foster [63, 64] in Hamster and Nicholson, [65] & Megahed [39] in birds, where they agreed that the (MT) consisted of 1) renal particles (RC).2) Proximal tubes (PT). 3) Distal tubes (DT). 4) Henley's bullish and falling lugs (HL). 5) Collecting tubes (CT). And it has a tissue structure similar to what was found in this study. Whereas, in the hamster, Sikri and Foster [63, 64]; Nicholson [65] in the whole of birds: described that the macula dense is considered the sign of the beginning of the tubes near the cortical nephron, and the cells of the dark spot are in closed contact with 1) - parent glomerulus. 2) Or with juxtaglomerular cells of different small arteries. 3) Or with both.
b) The effect of haloperidol on kidney tissue (Treated Groups)
In the current study, the data showed a clear delay in growth (retardation) for example delayed the appearance of the (MT) in the group (T1) did not appear on the 7 days, and consequently delayed the appearance of the (G) in the treatment (T0 and T1) at the age of 10-day. At this point, we supported what Bartolome et al. [66] said that the injection of neuro medicines during growth causes a temporary delay in brain development and therefore developmental delays.
Several works carried out by researchers were unanimous that early exposure to anti-dopamine neurodegenerative drugs leads to the closure of cell growth and maturation, which in turn leads to developmental delays [67-70].
Lu [71] proposed that the exposure of female mice to anti-neurological drugs during pregnancy, especially during the period of organ formation or fetal development and maturity, leads to delay and dysfunction of one organ to more in addition to the functional abnormalities of these organs. Moreover, Some authors [70, 72] reported that all neuro drugs lead to deficiency and delay in growth and distort fetal development and added that they lead to a deficiency in DNA and protein -contents, lack of cell count. Farhoud [73] also reported that exposure to haloperidol led to delayed growth of the embryos (testes) of mice and this delay was represented by the lack of growth and small size of semeniphrous tubule and lack of sperm formation.
Developmental delays were also detected by some researchers [8, 33] after the administration of antidepressant drugs. Thus, developmental delays of weeks or months since the onset of anti-neurological treatment were mostly caused by cell damage and destroy in pancreatic islands.
[74, 75]. Thereafter, Awad [76] confirmed that haloperidol led to a delay in the growth of the pancreas in the chick embryos in the 8,10,12-days, the Langerhans Islands did not appear on the 8-day, and this delay continued until the 15-day where the few appeared from the Langerhans Islands, and the beta and alpha cells were inferred on the 18th day with difficulty due to their late growth.
Some researchers found that Haloperidol induced retardation of growth of the hepatic tissues [77] and in the retinal tissues [78] The sign of retardation was investigated in the treated tissue of chick embryos which injection at the (zero-day) more than which injected at (5th days) of incubation.
Also, Tolba [79] added that the exposure of mice to fluoquitin hydrochloride with oral therapeutic dose from the seventh to the 21st day led to the apparent delay in testicular growth.
- In the present study, there were several degenerative changes represented in edema; congestion, and hemorrhage; vacuolar degeneration; fatty infiltration; fibrosis; cellular necrosis, and cell death, among the forms of nuclear decomposition (KL, and PK). In the T0, T1 class from the age of 7-10 days, and with the progress of growth and at ages of 14-16 days in the class T0, T1 the manifestations of decomposition increased in both (RC) and (RT) in the (MS) and (MT) the two-class were T0 then T1 more The affected groups. These changes are agreed upon by the finding of Neubert et al. [80], they noted that the neuron-drugs that are cause imbalance of the growth and differentiation of different tissues and cells in the embryos of mice after damage to these cells. Also, Megahed [39] shown that kidney cells lose their form, with many necrosis tissues Between the tubes, as growth progressed, the signs of decomposition were more pronounced and visible and included all the tubes and (RC) (Fig. 2,3).
Some scientists [81] confirmed that the (antidepressant) in humans led to increased activity, increased hepatic cellular decomposition, and increased fat deposition in liver cells and these changes are similar to those occurring in the liver of mice with increased In dogs. In the same way, some authors [82] stated that the administration of anti-nerve drugs to mice causes different pathological tissue changes in different organs such as; degenerative changes, pyknosis, necrosis a cellular infiltration of the interstitial lymphocytes.
The toxic effect of antidepressant drugs influences the formation and growth of the uterine wall and thus affects the process of fetal inactivity and consequently leads to abortion. It also causes serious phenotype and functional changes in hepatic fat with many degeneration changes [6, 71]. Also, several side effects were observed after treatment with haloperidol drug as antidepressants such as degeneration of the cells, the retina appeared to decrease in thickness, and less cell density with degeneration its cells [78] and also, on the retinal tissues [83].
A significant increase in brain dopamine and prolactin serum levels was observed by many researchers after the administration of antidepressant drugs [84-86]. There is also the decomposition of the epithelial in the seminal tubes, and in the testicle, there was a loss of all stages of growth of sperm where haloperidol led to the degenerating of testicular cells and ledge cells in treated mice. Cotran et al. [87] stated that the presence of edema as a form of decomposition, which is fluid between cellular or between the tissue, or the presence of vacuoles is caused by acute inflammation of the tissue in response to the damage caused by it, while Behl et al. [88] said that haloperidol is an anti-dopamine receptor and that as an active neuro-drug it has shown toxicity to primary neurons and caused death cells.
Recently, Kehinde [89] found that the HAL induced toxicity and suggest some possible mechanisms of action by which FO prevent HAL-induced hepatic toxicity in rats.
Moreover, Imanishi et al. [90] demonstrated that taking haloperidol showed Clear changes in the testicle (e.g. necrosis of sperm cells with atrophy of ledge cells, as some of the seminal vesicles were with pyknotic epithelial cells. Thus, it appeared that both the ability of the male organ and histological structures were affected. Burkitt et al. [91] attributed the occurrence of inflammatory reactions to the body's immune reaction against the damage caused by chemicals, viral and parasitic infections, and harmful factors to cells and tissues of the body.
The study carried by Curran [92] revealed that cell leaching of the cells of the eating and lymphatic is a sign of chronic inflammation where the eating cells devour the damaged tissues while lymphocytes produce antitoxins and speed up cellular repair processes. Also, Mienie et al. [31] explained the presence of necrosis and degenerative changes to the presence of the haloperidol compound (HPTP) and/or metabolites, causing a lack of cellular energy production in monkey tissue cells, because the drug prevents respiratory mitochondria from performing their work.
However, some investigators [73, 93] showed that haloperidol led to the increased thickness of the capsule coated to the testicle and its irregularity with the observation of many manifestations of cellular decomposition and necrosis on the testicular cells.
One of the drawbacks of antidepressant (anti-neurological) drugs [94, 95] is represented in inducing diabetes which may be caused by the breakdown and decomposition of pancreatic cells. Otherwise, Overstreet et al. [96] which discussed the subacute dose leads to progressive breakdown of liver cells, while a preliminary examination shows that about 50% of hepatic cells appear necrosis.
Pancreatic cell growth and subsequent pancreatic damage occur within 6 months in people using anti-neurological drugs, and that the affected cells appear in 12% of individuals treated with haloperidol [97]. The Haloperidol drug administration causes oxidative stress products, which in turn lead to cell death, as well as nuclear degradation and cellular necrosis, as haloperidol has a direct role in the creation of cellular decomposition [98, 99]. Moreover, Awad [76] confirmed that a large amount of decomposition and awful changes. As the 15-18 days increased the manifestations of decomposition and necrosis. Also, Marchese et al. [100] acknowledged that haloperidol was a cause of reduced immune system and shrinking of the bodies of black dopamine cells containing dopamine. Nearly, in the same way, some investigators [79, 101-103] demonstrated that injecting mice with haloperidol leads to a moral decrease in the level of the male hormone, in addition to a decrease in the size of the epithelial and seminal vesicles and prostate.
Also, some researchers [92, 93] found that the degeneration of myelin, scarce microglial macrophages, expansion of nuclear intermembranous space, degenerated mitochondria, and vacuoles. Also, cytoplasmic swelling, with lysosomes and apoptotic bodies. The haloperidol may lead to damage in neurons via the necrotic process in both low- and high-dose applications.
- In the current work, cells in the drug treatment tissues have appeared far apart and separated with the spread of interstitial edema and aneurysm, (especially in the T0 aged 14 and 16 days, T1 at the age of 16 days, this observation was supported by Hassan [82] who stated that antidepressant medications cause various degenerative changes in the lung, heart, and muscles such as vasodilation, cell necrosis and the presence of intercellular swelling. Both Wheater et al. [104] and Stevens et al. [105] explained that the etiology of swelling caused by intermediate chemicals released after tissue damage is caused by increased permeability through the walls of blood vessels, which thus leads to plasma leakage to the surrounding tissues, leading to the formation of fluid exudates or so-called "edema" swelling (Fig. 6).
Furthermore, some workers [73, 79] stated that when testicular examination of 3-day-old rat babies whose mothers had taken haloperidol it was found that the seminal vesicles appeared very far apart and separated from each other with few numbers.
Conclusion
Haloperidol has dangerous effects on kidney development with powerful toxicity on the cells, and the effect induced by the drug depends on the time of administration.
Key of abbreviatons :
Mesonephros (MS); Metanephros (MT),glomerular (G), Bowman capsule (B.C), parietal layer (P.L), vesicular layer (V.L), capsule space (C.S.), blood sinuses (B.S), proximal tubule (PT), distal tubule (DT),brush border (b.b), mesenchymal tissue (M.C), metanephrogenic blastema (M.B), internal region (I.Z), external region (O.Z), collecting tubes (C.T), middle duct (M.D), central vein (C.V), ureter (U), vesicles (V).
References
