DESIGN AND PHARMACOCHEMICAL DEVELOPMENT OF NEW ANTHELMINTICS WITH BENZIMIDAZOLYL ARYLPROPENONE PROFILE: STRUCTURE-ACTIVITY RELATIONSHIP
Ouattara Mahama1*, N'Guessan Deto Jean-Paul1, Coulibaly Songuigama1, Sissouma Drissa2, Koné W Mamidou 3
|
|
|
Abstract
On the basis of our previous study, which had demonstrated the strong anthelmintic potential of benzimidazole-2-arylpropenones, we proposed to extend the anthelmintic evaluation by testing anovel series of benzimidazole against Haemonchus contortus. The aimof this work is to appreciate the impact of the bioisoteric replacement of arylpropenone by arylacrylonitrile.Furthermore, we have established the structural elements necessary to get good anthelmintic activities in series of 2-substituted benzimidazoles. The structure-activity relationship studies made after all biological evaluations gathered, revealed that in series benzimidazolyl-phenylpropenones, excellent anthelmintic activities similar to those of Ivermectin have been achieved with the unsubstituted phenyl derivative, with the metahydroxyphenyl derivative, and by replacing the benzene homocycle with a heterocycle like pyridine or furan. Of all the variations made around the benzimidazole heterocycle, it appears that the introduction of a chlorine atom on one of the potential sites of metabolism of benzimidazole (C-5), coupled with the non-substitution of pyrrolic nitrogen, is favorable for obtaining powerful anthelminthic activities superior to those of Ivermectin and similar to those of fenbendazole. The isosteres of the arylpropenone chain, namely arylacrylonitrile and aryl-α-cyanopropenone or even its cyclized derivative (arylcyclohexenone), did not allow the enhancement of anthelmintic activities expected.
Keywords: Benzimidazole. Arylpropenone. Arylcyclohexenone. Arylacrylonitrile. α-Cyano arylpropénone.Structure-activity relationships. Anthelmintic. Haemonchus contortus
Introduction
Both human and veterinary helminthiasis continues to be a growing issue in health care and at the socio-economic level. Different antiparasiticcompounds have been used until now [1, 2]. In fact, with the resurgence of the resistance of antiparasitic drugs [3-5], we are witnessing a significant increase in morbidity and mortality associated with helminth infections. The medication currently used for these parasitic diseases is limited by the upsurge of drug resistance to anthelmintics(Figure 1) [6-10].Therefore, there is considerable interest in the development of new anthelminticcompounds that are more effective and better tolerated. Interestingly, our previous pharmacochemistry studiesestablished the promising anthelmintic efficiency of benzimidazole-based arylpropenones against Haemonchus contortus [11]. This greatly comforts us to pursue with particular interest the investigations of biological activities around the benzimidazoles heterocycle. Herein, we designedby a conformational blockage of propenone,cyclohexenones linked to the benzimidazole ring, to mimic the cyclic structure of flavonoids from which chalcones are derived(Figure 2) [12]. Moreover, the advent of Closantel in 1977 followed by Monépantel in 2008 in human antiparasitic therapy, highlighted the interest of the acetonitrile group as a modulator of anthelmintic activity [13-16]. The addition of this group, to the pharmacophore styryl present in tetrahydropyrimidines anthelminthicslike Pyrantel [17], has led us to new modulators of activity namely acrylonitrile and α-cyano propenones. (Figure 2). Thus, in this work we prepared andevaluatedlarvicides activities of these four chemical profiles with benzimidazole supportsnamely 2-arylpropénone, 2-cyclohexenone, 2-arylacrylonitrile, and 2-α-cyanoarylpropenone (Figure 3).By grouping all the results of anthelmintic activities of 2-substituted benzimidazole derivatives against Haemonchus contortus, we aimed to seek new benzimidazole anthelmintic modulators such as methylcarbamate, thiomethyl or thiazolyl. Precisely, we highlighted in a structure-activity relationship study, structural elements favorable to the induction and improvement of anthelmintic activities, both in series of 2-arylpropenone benzimidazoles and in series of 2-arylacrylonitrilebenzimidazoles.
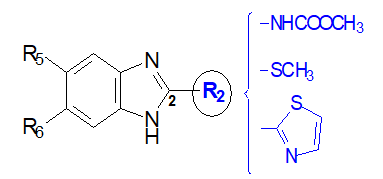
Figure 1: Old anthelmintic benzimidazoles and their pharmacophores in position 2.
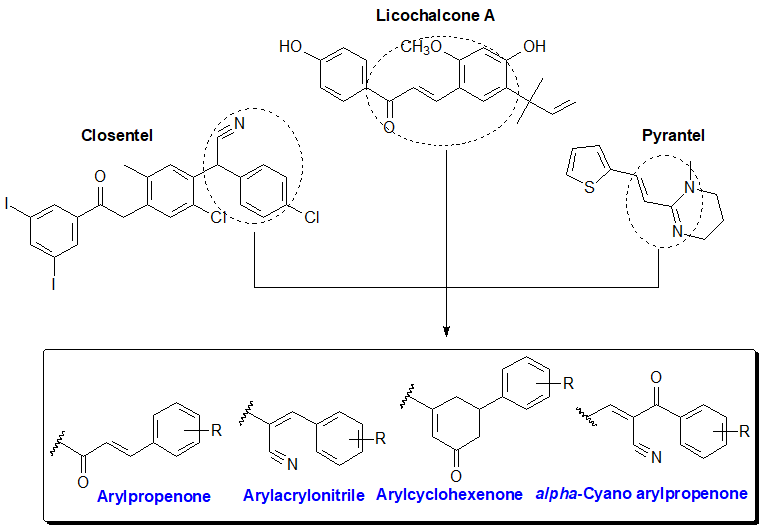
Figure 2: Design of potential anthelmintic pharmacophores.
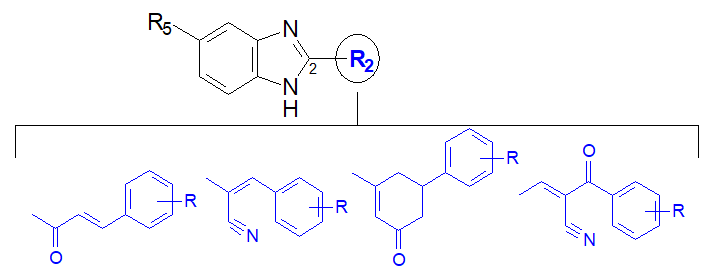
Figure 3: New potential anthelmintic benzimidazoles and their new pharmacophores in position 2.
Materials and Methods
Chemistry
The descriptions of the NMR spectra, mass spectra, and melting points for all compounds were described in previous studies [11, 18-20]. The thin layer chromatographies (TLC) were performed on silica plates Macherey-Nagel Sil G/UV254 or aluminaMacherey-Nagel ALOX N/UV254. Revelation of products was performed under a UV light source (254 nm). The solvents and reagents including benzaldehydes used were purchased from Acros Organics (France) or Aldrich (France). Standard antiparasitic drugs, Fenbendazole, and Ivermectinwere purchased from SIGMA Chemical Co. (USA).
General method for synthesis of compounds: Formation of benzimidazolyl-arylpropenonesand its cyclohexenone derivatives(7a to 7u)
Step1: Formation of the 2-acetylbenzimidazoles4a-e.
A mixture of orthophenylenediamine suitably chosen 1a-e (42 mmol), and lactic acid (2) (60 mmol) was stirred in 4N hydrochloric acid (50 mL), then heated at reflux for 45 mins. After the completion of the reaction (monitored by TLC), the cooled reaction mixture was alkalinized using ammonia. The solid obtained was filtered off, washed with water, and recrystallized with water. The desired product 2- hydroxyethylbenzimidazole (3) was isolated in high yields in the essentially pure form.To a solution of 2- hydroxyethylbenzimidazole (22 mmol) in 30 mL of acetic acid was added 10 mL of an aqueous solution of potassium dichromate (11 mmol). The reaction mixture was heated under reflux for 45 min. The precipitate afforded after cooling and neutralization with ammonia was filtered off and then taken up in chloroform. The organic layer was then washed with water, dried over magnesium sulfate and then evaporated under reduced pressure. The solid of 2-acetylbenzimidazole and its derivatives4a-ewere obtained in an overall yield of 72% after recrystallization in water/Ethanol (1:1) [19] (Scheme 1).
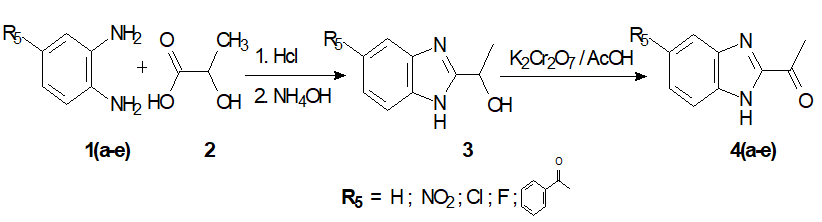
Scheme 1: Synthesis of 2-acetylbenzimidazole and its derivatives C5 substituted
Then, from methyl ketone 4a, we carried out anN-benzylation reaction using benzyl chloride and sodium hydride. As a result of purification by recrystallization, the benzyl derivative 4f was isolated in 71% yield(Scheme 2) [18, 19].
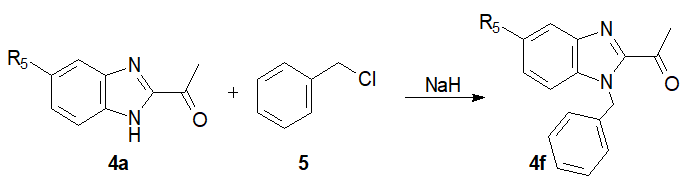
Scheme 2: Synthesis of N-benzyl 2-acetylbenzimidazole.
Step 2: Formation of benzimidazolyl arylpropenones C-5 substituted7a-t.
To 2-acetylbenzimidazoles 4a-f (10 mmol), was added arylaldehyde 6a-m (10.1 mmol) properly chosen and the mixture was stirred in an ethanolic solution of sodium hydroxide (75 mmol of sodium hydroxide in 40 mL of ethanol) at room temperature for 3 to 5 hours. The alkalinization of the middle using a solution of acetic acid to 30% afforded a precipitate. The achieved precipitate was filtered, dried, and recrystallized to yield benzimidazolyl arylpropenones 7a-t (50-86%) as crystals (Schema 3) [11, 18, 19].
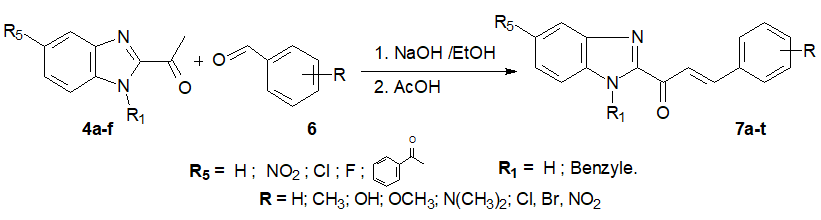
Scheme 3: Synthesis ofthebenzimidazolyl arylpropenones C-5 substituted.
Step 3: Formation of benzimidazolyl arylcyclohexenone7u.
Benzimidazolyl arylpropenone 7a and ethyl acetoacetate 8 in the 1:1,2 ratio in 15 mL of ethanol were maintained in a crushed ice bath at -10°C for 2 h. Then, 0.1 mL piperidine was added and the reaction mixture was kept overnight at room temperature. The precipitate was filtered off and recrystallized from ethanol/toluene mixture (1:4) to yield a pure solid of cyclohexenone 7u as diasterioisomeric mixture with 83% yield(Scheme 4) [19].
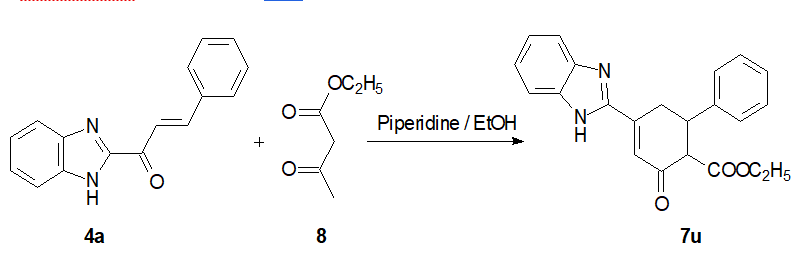
Scheme 4: Synthesis of benzimidazolyl arylcyclohexenone.
General method for synthesis of 1H-Benzimidazol-2-ylacrylonitrile (12a-h) and its analog phenyl α-cyanopropenone (14a)
Step 1: Synthesis of 1H-Benzimidazole-2-acetonitrile11.
The mixture of o-phenylenediamines 1a (4.2 mmol) and ethylcyanoacetate 10 (7 mmol) washeated for 2 h at 200°C. After cooling, the residue was extracted with chloroform and purified by column chromatography using EtOAc/n-hexane (1:1) as eluant and decolorized with activated carbon.1H-Benzimidazole-2-acetonitrile 11 was afforded with an acceptable yield (35%)(Scheme 5 ) [20].
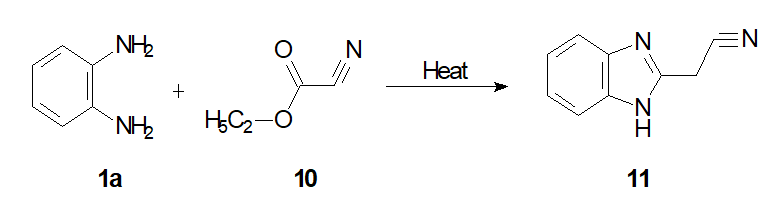
Scheme 5: Synthesis of 1H-benzimidazol-2-ylacrylonitrile.
Step 2: Synthesis of the 1H-Benzimidazolyl-2-acrylonitriles 12a-h.
To a solution of 2-cyanomethyl benzimidazole in absolute ethanol (20 mL), equimolar suitable chosen benzaldehyde derivative and few drops of piperidine were added. The reaction mixture was heated under reflux for 2–6 h. Then cooled to room temperature and excess of ethanol wasevaporated at reduced pressure. Freshlydistilled water was added to the crude mixture and the precipitatedproduct was filtered and dried. The pure product 12a-h was obtained by recrystallizationfromethanol (Scheme 6) [20].
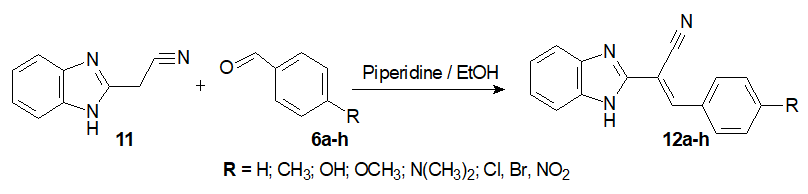
Scheme 6: Synthesis of the 1H-Benzimidazolyl-2-acrylonitriles
Step 3: Synthesis of 1H-Benzimidazolyl-2-phenyl α-cyanopropenone 14a.
A suspension of 2-cyanomethyl benzimidazole 11 (1.5 mmol.) and benzoyl chloride 13 (3 mmol) in dry toluene (20 mL) was heated under reflux for 5 h. The reaction mixture was cooled to 20°C before filtration. Theresidue was then washed with water (3 x 5 mL),then crystallized from acetic acid and dried yielding benzimidazolyl phenyl α- cyanopropenone 14a ( 60%)(Scheme 7) [20].
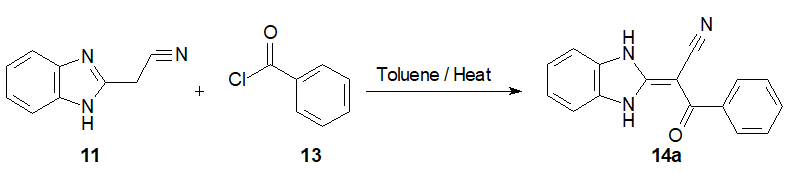
Scheme 7: Synthesis of 1H-Benzimidazolyl-2-phenyl α-cyanopropenone
Anthelmintic activities
The in vitro screening of anthelmintic activities of 21 arylpropénones derivatives, 9 arylacrylonitriles derivatives,and structural analogs was carried out on a helminthic strain of Haemonchus contortusfurnished bySwiss Center for Scientific Research in Côte d'Ivoire by the Larval Development Assay (LDA) [21-23]. The tests were conducted in triplicate with all the products that showed activity. The tests were performed compared to two reference drugs namely Fenbendazole and Ivermectin.
Results and Discussion
The results obtained at the end of theevaluation of antilarval activity againstH. contortus of the new benzimidazole derivatives, the Fenbendazole and Ivermectin gathered in Tables 1, 2, and 3. The activity of each product is given by its larvicidal concentration 100 (LC100 ) expressed in micromolar (μM).
Table 1: In vitro anthelmintic activities of reference substances against Haemonchus contortus.
|
Reference substances’ names |
LC100 (μM) |
|
1,3-diphenylpropenone (Chalcone) |
57.210 |
|
Fenbendazole |
0.002 |
|
Ivermectin |
0.010 |
Table 2: In vitro anthelmintic activities of benzimidazolyl-arylpropenones and structural analogs (7a-u) against Haemonchus contortus.
|
Chemical structure |
Compoundcode |
R |
LC100 (μM) |
|
|
7a |
H |
0.007 |
|
|
7b |
2-CH3 |
43.220 |
|
|
7c |
3-CH3 |
10.310 |
|
|
7d |
4-CH3 |
0.574 |
|
|
7e |
2-OH |
10.240 |
|
|
7f |
3-OH |
0.007 |
|
|
7g |
4-OCH3 |
2.310 |
|
|
7h |
2-Cl |
0.535 |
|
|
7i |
4-Cl |
40.260 |
|
|
7j |
3-Br |
0.466 |
|
|
7k |
4-F |
42.610 |
|
|
7l |
3-NO2 |
0.030 |
|
|
7m |
4-NO2 |
9.278 |
|
|
7n |
- |
0.007 |
|
|
7o |
- |
0.008 |
|
|
7p |
H |
˃33.940 |
|
|
7q |
Cl |
0.002 |
|
|
7r |
F |
0.566 |
|
|
7s |
|
8.144 |
|
|
7t |
NO2 |
˃41.02 |
|
|
7u |
H |
32.130 |
Table 3: In vitro anthelmintic activities of benzimidazolyl-arylacrylonitriles and structural analog against Haemonchus contortus (12a-h, 14a).
|
Chemical structure |
Compound code |
R |
LC100 (μM) |
|
|
12a |
H |
46.39 |
|
|
12b |
CH3 |
˃44.01 |
|
|
12c |
OH |
˃43.69 |
|
|
12d |
OCH3 |
˃41.58 |
|
|
12e |
N(CH3)2 |
˃39.78 |
|
|
12f |
Cl |
˃40.95 |
|
|
12g |
Br |
35.57 |
|
|
12h |
NO2 |
˃39.53 |
|
|
14a |
H |
43.69 |
The results of the relationship between structure and activity revealed that the introduction in position 2 of the benzimidazole nucleus of the arylpropenone functional chain of chalcones to obtain a benzimidazolyl-phenylpropenone 7aallowed to induce anthelmintic activities of 0.007μM. It appears that our design undertaken,made possible to obtain a remarkable anthelmintic compound.
The arylpropenone group can, therefore, be considered as a new anthelmintic performance modulator, like the methylcarbamate, thiomethyl or thiazole of traditional benzimidazole anthelmintic [24]. This result corroborates the intrinsic anti-infectious (antiviral, antibacterial, antifungal, and antiparasitic) potentials of the benzimidazole ring and the arylpropenone group of chalcones [25-27]. Moreover, the anthelmintic activity of some compounds derived from benzimidazole 2-phenylpropenone 7a was found to be dependent on the substitution at different levels of its structure (Figure 4).
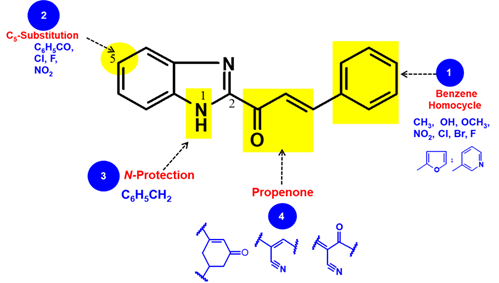
Figure 4. Pharmacomodulation sites of benzimidazolyl arylpropenone.
Structural variations of the benzene homocycle.
The structural variations around the benzene homocycle of compound 7a led to the following findings:the substitution of a homocyclic ring with a methyl group leads to a decrease of the anthelmintic activities observed with compound 1a (LC100 = 0.0075 μM) whatever the isomeric position of this group is. In addition, thelarvicidalactivities of all methylated derivatives remain insufficient compared to those of references namely Ivermectin (LC100 = 0.0103 μM) and Fenbendazole (LC100 = 0.0017 μM).
The orientation of the hydroxyl group proved to be important, as when it is in the meta position. Indeed, only the meta hydroxylated derivative 7f,with an activity of 0.007 μMshowed anthelmintic activity similarto those of benzimidazolyl phénylpropénone 7a. In contrast, the O-methylated derivative in the para position 7g did not achieve an anthelmintic activity as good as its m-hydroxy analog.The chlorination in ortho or para position of benzene homocycle of 7a (7h and 7i respectively) led to fewer contacts to residues in the binding pocket with a decrease in the larvicidal activity of up to 40 μM. Replacing the chlorine in para position 7iwith a fluorine 7kmaintains activity around 40 μM. With an anthelmintic activity at 0.5 μM, the meta-brominated derivative 7j seems to fit in the target pocket, suggesting that the electro-attractive effect of the halogen group is likely to have a positive influence in the ortho or meta position of the benzene homocycle.
Likewise, the electron attractive property of the nitro group (7land 7m), is most likely tohave relevance in improving biological activity in the meta position of the benzene ring(LC100 = 0.03μM). This activity, remain beneath those of the non substituted derivative. Finally, the replacement of the benzene homocycle with a heterocycle of pyridine 7nor furanic 7oled to the maintenance of anthelmintic activity around0.007μM. Such efficiencies are indeed superimposable with those of benzimidazolyl-phenylpropenone )about 7nM(. It emerges from all the aforementioned modulations carried out around benzene homocycle that obtaining excellent anthelmintic activities superimposable on those of Ivermectin is based either on non-substitution of the benzene homocycle, or on the presence of a meta hydroxyl group on the benzene homocycle, or on replacementof the benzene homocycle with a pyridine or furanic heterocycle.
Structural variations of the benzimidazole heterocycle.
Anthelmintic screening of derivatives resulting from the C5 modulation of benzimidazole suggested that the presence of a benzoyl group (7s) as in the Mebendazole molecule, or a nitro 7t, leads to a decrease or even a total loss of anthelmintic efficacy. Indeed, with LC100 respective of about 8 and up to 41μM, the anthelmintic performance of these derivatives was much lower than those of benzimidazolyl phenylpropenone.In addition, It appears that the chlorine atom at the 5-position of the benzimidazole ring 7q has increased by about 4 times the anthelmintic activity of that of its non-chlorinated analog 7a. This modulator in the molecule of Triclabendazole [28] induced remarkable activity)up to 1.7 nM(. This anthelmintic efficacy is superimposable to that of Fenbendazole and is about 5 times more active than Ivermectin. Notwithstanding, the replacement of chlorine by Fluorine 7r did not enhance the anthelmintic activity.
Considering only the difference in N1, it would be able to deduce that bulky groups like the benzyl group in the pyrrole nitrogen of benzimidazole nucleus (7p)areunfavorable to the activity (LC100 > 34μM). This absence of activity confirms that free pyrrole nitrogen is essential to induce good activity in a series of anthelmintic benzimidazoles drugs [24]. Based on all the foregoing variations, it appears that the introduction of a chlorine atom on one of the potential sites of metabolism of benzimidazole, coupled with the non-substitution of pyrrolic nitrogen, is favorable to obtain powerful anthelminthic activities superior to those of Ivermectin and superimposable to those of Fenbendazole.
Structural variations of propénone.
In the first place, it was cyclized to cyclohexenone 7u like ethyl 4-(3-bromophenyl)-6- (6-methoxy-2-naphthyl)-2-oxo-cyclohex-3-ene-1-carboxylate which has shown interesting anti-infectious activities in vitro [27]. Such a variation, which introduces a conformational restriction, has not been shown to be appropriate for improving the anthelmintic activities of the cyclohexenone derivative, whose concentration is 32 µM. In a second modulation, we replaced propenone with acrylonitrile through the bioisosteric substitution of −CO by -CN, while maintaining the previously introduced modulators of benzimidazolyl arylpropenones [28]. Since all the tested acrylonitrile derivatives 12a-h showed no-to-moderate anthelmintic activity against Haemonchus contortus, it can be inferred that the addition of additional carbon on the α,β-ethylenic chain is unfavorable to the interaction with the biological target. As far as we know that some α,β-unsaturated nitrile-containing drugs are conjugated with additional electron-withdrawing groups to facilitate Michael additions as in entacapone [26], we further designed the α-cyanopropenone resulting from the introduction of the nitrile adjacent to a hydrogen bond acceptor, a ketone group. Surprisingly, This derivative was less effective than propenone isostere. Like previously, this suggests that the concept of bioisostery did not ameliorate anthelmintic properties. As a result ofthe previous modulations around propenone, the cyclization thereof in cyclohexenone or its replacement by acrylonitrile or an α-cyanopropenone is not propitious to the improvement of anthelmintic activities.
Conclusion
Interesting trends have been observed in structure-activity relationship studies carried out from the anthelmintic activities of 2-substituted benzimidazole derivatives against Haemonchus contortus.These findings can be summarized in three majorpoints. Firstly, the presence of chlorine-type halogen at position 5 on the benzimidazole nucleus, doubled with the presence of pyrrole nitrogen of a secondary nature, may be considered a real scaffold for anthelmintic activities. The substitution of the −NH− group in the benzimidazole ring by a hydrophobic benzyl group drastically decreased the anthelmintic activity. Secondly, the arylpropenone functional group at the 2-position of benzimidazole would be an excellent modulator of anthelmintic activities such as methylcarbamates and thiomethyls of anthelmintic benzimidazoles used therapeutically. Finally, nitrile does not function as a ketone bioisostere, which proves that the ketone group in the arylpropenone chain is likely to be involved in specific, non-polar interactions with the target. Similarly, aryl-α-cyanopropenones or aryl cyclohexenonederivatives exhibited very weak larvicidal activity against Haemochus contortus.
Acknowledgments
The authors thank the Swiss Center for Scientific Research in Ivory Coast (CSRS-CI) for carrying out anthelmintic tests, and the CEISAM Laboratory of the University of Nantes for NMR and MS spectra.
References
