FROM HYDROTHERAPY TO THE DISCOVERY OF THE GUT MICROBIOTA: THE HISTORICAL GASTROINTESTINAL HEALTH CONCEPT
Lucrezia Bottalico1, Francesca Castellaneta2, Ioannis Alexandros Charitos1*
|
|
|
ABSTRACT
Aim of the study: this review aims to deepen, in the light of current knowledge on the essential physiological functions of the gut microbiota on human health, if the so precious role of this gastrointestinal ecosystem has been identified since ancient times and what how past society supported its correct functionality. Methods: Medline - PubMed, CDC website, Scopus and WoS databases were searched to identify relevant studies on the history of gastrointestinal medicine in the past, particularly we included studies on the importance of bacterial flora on human health. Results: medical knowledge on the important benefits of the gastrointestinal bacterial flora on the whole human body dates back to very ancient times. Many evidences suggest that ancient scholars from past Asian, African and European civilizations well-understood the importance of preserving gastrointestinal ecosystem for maintaining human health, from the sophisticated drainage systems of waste water from houses and public places since today when analysing in detail functions and composition and health’s advantages of the gut microbiota’s correct functionality is making great strides. Conclusion: the close relationship existing between the gut microbiota and the host has always had a great attention in the course of history. The most recent discoveries in understanding mechanisms of gut microbiota underline its importance in preserving host health, that past civilizations cleverly understood.
Keywords: Gut microbiota, gastrointestinal ecosystem, human health, hydrotherapy, history of medicine
Introduction
The abundance of microorganisms colonising the human gastrointestinal (GI) tract, estimated to exceed 1014, is termed the “gut microbiota”[1, 2]. These commensal microorganisms have a vital role in human health: they represent one of the main the beating heart of several important biological and physiological functions, in an intricate and mutually beneficial relationship with the human host with which it has co-evolved over thousands of years [1, 3]. Before the discovery of the human gastrointestinal microbiota composition and its impact on gut integrity and host health, the potential benefits of an efficient bacterial flora has been investigated for many centuries and cleverly deduced by past civilizations that stressed and emphasized the importance of a healthy gastrointestinal ecosystems through proper nutrition, adequate hydration and a structured sewerage system to treat and channelled waste water.
Materials and Methods
Methods:
Medline - PubMed, CDC website, Scopus and WoS were extensively searched for relevant studies published on the history of gastrointestinal medicine in antiquity, especially regarding the knowledge by ancient scholars about the complex role of the bacterial flora on the whole human body and the intestinal and extra-intestinal consequences of its alterations. In addition, we included historical books on the topic of interest and original sources from Greek historical archives. Papers with historical relevance investigating knowledge on gut’s ecosystem impact on the host health by ancient physicians to the more recent founds on the adult human gut microbiota were included. We searched literature published in English, including items translated into English from a foreign language (Chinese, Egyptian, Indian, Greek and Latin).
Results
Improvement in the Sewer System at the base of a Healthy Society
It is difficult to identify the exact moment in history that hydrotherapy and particularly the internal colon bathing therapy procedures have been used. The development of the sewer in history from those earliest of civilizations through to the present day gave a vital contribution to human gastrointestinal and systemic health. Since ancient times, people understood the importance to avoid human diseases of removing bodily waste with sophisticated drainage systems from houses and public places. First evidence of superficial drainage systems was found in Iraq, dating back ca. 4000–2500 BC during the early Babylonian and Mesopotamian Empires [4, 5]. During the 1930s, the findings of the Mari Palace in Mesopotamia was equipped with baths. In the history of Assyrian and Babylonian civilization, the title of physician was A-Su, that is meaning “knowledge of water”. In some cuneiform inscriptions were found on tablets as early as 600 b.C. they probably describe bathing with colon hydrotherapy and its simple form the ἔνεμα (enema =To throw in) practice. Also, in the culture of Mohenjo-Daro (modern-day Pakistan), which begins at the end of the 4th millennium b.C. or early 3rd, excavations revealed platforms that supported large brick buildings, and smaller ones, which belonged to for homes which had a bathing facility. Even though other ancient civilizations also contributed, notably some of the Chinese dynasties. [4-6].
In the writings of the ancient Egyptians and precisely in the Ebers Papyrus (1550 b.C.) we have descriptions with the various methods of preparation and execution of enemas as a remedy for disorders in the stomach and intestines and “to drive away the bad excrements”. Furthermore, the administration of drugs through the rectum is also mentioned in the same papyrus. According to the Latin Pliny the Elder (Gaius Plinius Secundus 23–79 a.C.), the god Thot introduced enemas and invented equipment and formulas for the various treatments. [4, 7, 8].
After 3000 b.C., evolved drainage technologies and sewers were built by the Minoan in Crete and the Harappan civilizations in the Greater Indus Valley. The bathing of the human body is an element of the religious ritual of Hindu culture and is still practiced with the bath in the Ganges River. In addition, the ancient texts of Indian civilization make and references through medical texts such as Caraka-Sambita and Susruta-Sambita that describe the practice of two main hydrotherapy procedures and the rectal speculum. [4, 7]
Later, the Greeks and Romans they have had attention both in urban sanitation and in developing elaborate sewerage and drainage technologies. For the Greeks, the construction of spas has its roots in the identification of their function of psychosomatic help and in the determination of their systematic use which was initially characterized as a creation of nature, that is, as a gift from the gods and as a means of medical treatment. [4, 6]. The most important researcher of the hydrotherapy was Hippocrates (Ἱπποκράτης, 460-377 b.C.), considered the father not only of medicine but also of bathing procedures where he practiced in the Asclepieion temple of Kos. He makes a clear distinction about waters: the drinking springs water (πόσιμα), stagnant (στάσιμα), rainwater (όμβρια) and those derived from rocks (πέτρινα). It he refers to cold or hot water that containing metals such as iron, copper, silver, gold, sulfur, alum and has particular healing properties. He had noticed the stimulating effect of warm mineral water on the human body, as well as the chemical effect of metals. Indeed, in his medical work “On Air, Waters and Places” (Περί αέρων, τόπων, υδάτων), it appears that he describes the influence or the waters on people's health and the hydrotherapy process. According to Hippocrates, too cold water could cause serious intestinal disorders, while too hot or stagnant water often causes fever. He also knew about the advantages of clean and warm water for the wound therapy and gave major therapeutic value to marine baths. In another his work the “Περί διαίτης οξέων” (On the diet during acute) Hippocrates describes the routine of bath therapy in way that does not differ from today’s one. He specifically mentions: “…Bath does good to many diseases, sometimes continually or even sporadically and used the purification of the rectum to treat feverish fever and diseases in general…”, according to his theory of “humoral theory” (θεωρία των χυμών). [7-11] In addition, mentions the probiotic sour milk and he considers it an excellent food with therapeutic properties for intestinal disorders, that later is confirmed in “Naturalis Historia” by Pliny the elder and the physician Galen (Γαληνός, 129–216), exposing the soothing and laxative effect on the intestines and for treating gastroenteritis.[12-14] What is important, however, Hippocrates classified many diseases to try to determine the healing effects of hot and cold baths. Finally, another element that adds Hippocrates is that the first that separates the thermal waters, based on their smell and color. In the 6th and 5th centuries b.C. medical art was practiced in the Asclepieions, which served as religious and therapeutic centers. The fact that most Asklepieions have been built near hot springs, rivers, hot springs or near the sea it was not occasional. The first medical measure applied to the patients of Asclepieion was various formulas bath for the purification of the ill patients. In addition to the Asclepieions there were physicians who knew the power heal of the waters and used it for therapeutic purposes outside of them. A sample of an important Greek center was the Asklepieion of Pergamon (500 b.C.), which had balneotherapy facilities, massages, gymnasium, one theater for 30,000 people and, of course, a large precinct with a length of 1,000 m. The renowned Asclepieion of Epidaurus is considered the main sanctuary of hydrotherapy such as the Asclepieion of Kos. [9, 11, 15, 16] Subsequently, the supporters of Hippocrates as the anatomists of the Hellenistic medical school of Alexandria in Egypt Herophilos (Ηρόφιλος 335-280 b.C.) and Erasistratus (304-250 b.C.) and later, during the Roman period the physician Asclepiades (Ἀσκληπιάδης, 124-40 b.C.), that he recommended the enemas and not the use of laxatives for worms and intestinal fevers, Claudius Galen (Κλαύδιος Γαληνός, 129-216 AD) who also recommended the use of various types of enemas with the use of honey and oil, Antonius Musa (Αντώνιος Μούσας, Ith century BC) and others. So many physicians where they recommended hydrotherapy, generally intestinal bathing and drinking thermal waters as an additional treatment for certain disorders and diseases along with diet and exercise. Enemas were mentioned also by the historian Herodotus and was also consisting of astringents, emollients, nutrients, anthelmintic and antispasmodics. Later according to Celsus (2th century a.D.), on the work “de Medicina”, he wrote: “….This remedy should not be repeated too often, nor should it be too hot or cold…. health and consequently malnutrition, as a weakened state leaves him exposed to diseases of all kinds…”.[4, 13, 14, 17-21]
At the Roman ages, the Bithynian physician Asclepiades laid the foundation of Methodiki (Μεθοδικη) medical school at Rome. It supports the baths facilities in particular cold baths (ψυχρολουσία: psyhrolousia) in particular for the neurological diseases. It was the first that introduced hydrotherapy and enemas to the Romans and he perfected the procedures for the participation in the baths, making this phenomenon also a high-level social event. Thus, the Romans, using the well-organized military and administrative faculties of their empire, used hot and cold natural sources, initially for the cleaning and training of soldiers, but also for the treatment of diseases. These facilities buildings are located both in the Italian area and in the conquered countries. In Rome, the first thermal complexes were built after the contamination of the Tiber River. The Roman emperors considered it necessary to build a thermal complex, which usually took their name. Thus, the first baths built on the glories of the emperors were Marcus Vipsanius Agrippa (63 -12 b.C.) and Titus (25-12 b.C.). Thus, during the Roman Empire, many thermal baths were built, some of which had a capacity for 1,500 people and 3,000 individual marble bathtubs. [21-24]
In the medieval times, the use of spas was limited only in the Roman Christian Empire (Byzantium). In addition, many Byzantine doctors advised enemas as Aetius Amidenus (Αέτιος Αμιδηνός, 6th century a.D.) and some of them tried to observe the diseases through the characteristics of the excrements. Indeed, the physician Theophilus Protospatharius (Θεόφιλος Πρωτοσπαθάριος,7th a.D.), who was the chief physician of the emperor Flavius Heraclius Augustus (575-641 a.D.) wrote the work “Περί διαχωρημάτων” (On feci) and at the 12th century, Joannes Εpiscopus of Ρrisdryanorum (Ιωάννης επίσκοπος Πρισδρυανών), he wrote the two works “Παρεκβολαί εκ των παλαιών ιατρών συλλεγείσαι περί διαχωρημάτων” (References from the old doctors collection about feces) and the “Εκ των Παλλαδίου, Αρχελάου, Στεφάνου Αλεξανδρέως και διαφόρων παλαιών ιατρών περί εντέρων” (From Palladius, Archelaus, Stephanus of Alexandria and others ancient physicians about intestines). Among the eminent Arabs, some doctors describe, perhaps for the first time, the syringe enema such as Avicenna (980-1036 a.D.) and Albucasis of Cordova (1013-1106 a.D.) in his work “Health Rectification”. Instead the Arabs subsequently influenced by the Byzantine spa adapted everything in their traditions by creating the hammam bath facilities. [5, 25, 26]
During the Late Middle Ages thermae and balneae facilities are no longer used instead the enemas procedures continued to grow. The English surgeon John Ardene (1307-1390 a.D.) wrote a treatise on enemas entitled "Treatise of fistula in anus, hemorrhoids and enemas" and later an innovation came from Ambroise Pare (1510-1590) with a syringe enema device for self-administration. During the 17th-18th centuries, the use of enema was widespread in Europe. In 1600 Louis XIV spreading its use also among ordinary citizens. To make the use of the enema even more usable, the Dutch doctor Reinier de Graaf (1641-73) at 1668 wrote the De clysteribus (On Syringes) and developed a simple and practical tool that consisted of controlling the syringe with a flexible hose at a stiff beak, which forms the final part. In addition to his invention, formulates 27 preparations ranging from purgatives to detergents, from nutrients to emollients. In the mid-18th century, Edward Jukes invented two types of enema apparatus units for internal colony bathing: the flexible clysmaduct and the pressure-fed. However, in the late 19th and early20th centuries, with the appearance of various pharmacological treatments and the wide use of laxatives (more comfortable) the use of colon hydrotherapy will also become less popular in the medical community, will decrease. Only later thanks to the physician John Harvey Kellogg (1852 -1943) that the internal bathing therapy has been renewed with the addition to proper nutrition. Since the 1940s, colon hydrotherapy equipment has continued to evolve and, in the early 1950s, flourished in the United States to "decrease until the early 1970s, in favor of colostomy, fleet enema and prescriptive laxatives. In adition, the colonic lavage with polyethylene glycol (PEG 3350) procedure is used for some intoxications such as the pharmacological one from lithium [5, 27, 28]
Finally, it was only from 1850 onwards that modern spas was “reborn”, but many of the principles grasped by the ancients are still valid today. Thanks to the physician and scientist (chemist, physiologist and anatomist) Franciscus de le Boë Sylvius (1614-1672) scientific research had begun, according to which the user or the patient needs a prescription, which will define both the quantities and the intervals of the drinking thermal water and are tested during this period with other therapies, such as mud baths, steam baths and special hydrotherapy with intense water splashing. It is based on theories and knowledge of Paracelsus and Matteo Bendinelli (1487-1530) on chemical analysis and minerality of water. The link of evolution to this came from the England of Robert Boyle, who is the founder of modern chemistry with the introduction to the physical-chemical perception of matter. The physician Johann Jacob Scheuchzer (1672-1733) began to distinguish the physical and chemical properties of water in cool or cold, acidic, saline, sulphurous and hot, which helped to associate them with certain disorders. In the and 19th century, new spas facilities are reformulated without, essentially, detaching themselves from the ideological model that had already been established in the ancient Greek and Roman ages. In contrast, the scientific foundation of therapies is validated through thermal waters and the era of social tourism begins in the 20th century. [4, 6, 29-31]
The discovery of the Human GI Microbiota
The main scientific step was in 1907, by Ilya Ilyich Metchnikoff, in his study titled “The Prolongation of Life” that he promoted the Lactobacillus acidophilus and the main metabolite of the fermentation of sugars, the lactic acid. Metchnikoff was the first who discovery the importance of lactobacilli in human health and longevity, thus their reduction was the responsible agent for the weakening of the intestinal system and aging. [12] The Human gastrointestinal microecology consists of 3 million species or over 100 trillion microorganisms), thus 400 species and 1014 bacterial cells. In the gastrointestinal district there are mainly 2 of the 55 Phyla known today (Firmicutes and Bacteroides) and about 15% of the more than 900 known species. The mouth and the all intestine contain the widest population of bacterial species and the stomach the least one (small intestine 104-106 Lactobacilli, Gram + cocci and Colon 1012/g of Bacteroides, Bifidobacter, Peptostreptococci, Fusobacteria, Lactobacilli, Enterobacteria, Enterococci, and Clostridia) (Tab1). [31, 32]
Table 1: The main prevailing microbial phyla in the various districts of the human body
|
Prevailing Microbiota |
|
|
Location |
Phyla |
|
Skin |
Actinobacteria, Firmicutes, Proteobacteria |
|
Mouth |
Bacteroides,Firmicutes,Fusobacteria,Proteobacteria |
|
Airwais |
Bacteroides, Firmicutes, Proteobacteria |
|
Gastrointestinal |
Bacteroides, Firmicutes, Actinobacteria |
|
Urogenital |
Firmicutes |
The gut microbiota provides many important benefits to the host, in a very close symbiotic and mutually beneficial relationship. First of all, these commensals, such as A.muciniphila [32] and Lactobacillus plantarum [33], help the host to the development and to maintain the integrity of the intestinal epithelium and of the mucosal barrier [33]. Species such as B. thetaiotaomicron and F. prausnitzii, A. muciniphila, R. gnavus E1 and Lactobacillus casei DN-114 001 are implicated in modulating mucus turnover, thickness and properties such as mucin glycosylation. [34, 35]. Gut microbiota also breaks down otherwise indigestible dietary fibres [36-38]; it provides the synthesis of essential vitamins which the host is incapable of producing (vitamin B12 by Lactic acid bacteria, folate mainly by Bifidobacteria, but also riboflavin, biotin, vitamin K, nicotinic acid, panthotenic acid, pyridoxine and thiamine [39-44] and carbohydrate-active enzymes which are able to ferment complex carbohydrates, generating metabolites such as propionate, butyrate and acetate [45, 46], which are involved in regulating several GI cellular functions, such as gene expression, chemotaxis, differentiation, proliferation, apoptosis [47-50] but they are also important energy source for colonocytes [26, 51-55]. Another fundamental function of Colonic bacteria is to metabolise unabsorbed bile acids for biotransformation to secondary bile acids [12, 28, 55]. It protects against environmental factors, antigens and, though the production of various antimicrobial compounds, it provide immune protection against pathogenic bacterial species, preventing from colonizing the gut and stimulating release of mucosal secretory IgA [56-58] in fact, it is able to influence pathogen colonisation competing for attachment sites or nutrient sources, and by producing antimicrobial substances [12, 59-61]. Its interaction with the host’s mucosal immune system helps in regulating its development and maturation and it is crucial for proper immune function [46, 48, 62]. For its involvement in all these functions, it is often referred to as a ‘superorganism’ essential for host health (Table 2) [12, 31, 48].
Table 2: The effects of the Human gastrointestinal microbiota in the Large intestine
|
MICROBIOTA'S ACTION IN THE LARGE INTESTINE |
|||
|
Deleterious |
Beneficial |
Combined |
|
|
Microbiota |
Clostridia Pseudomonas aeruginosa Proteus Staphylococci Veillonellae |
Lactobacilli Bifidobacteria |
Bacteriodes Enterococci Streptococci E. Coli Eubacteria |
|
Effects |
|
|
|
Gastrointestinal microbiota starts to develop from birth, when the GI tract is rapidly colonised by bacteria. The infant's intestine at birth is sterile. In the first days of life there is a colonization peculiar to each child and dependent on the type of birth (vaginal delivery lead in oral inoculation with intestinal and vaginal bacteria: Lactobacilli, Prevotella, Sneathia, E. coli, Clostridium difficile, Bacteroides fragilis and with the cesarean delivery: Staphylococcus, Corynebcterium, Propionibacterium). In addition, cesarean delivery is associated with a reduced number of Bifidobacteria and B. fragilis and an arise of Clostridium difficile. At four days the microbiota of vaginal delivery babies resembles the maternal vaginal one and the cesarean from the mother's skin microbiota. [63, 64] On an individual level there is a notable difference from child to child in the temporal profile of colonization, which is instead very similar in twins. From the 5th day of life onwards, the profile of the microbiota begins to resemble that of an adult [65]. At one month all babies are colonized mainly by bifidobacteria, E. coli and B. fragilis, less by Clostridium difficile and lactobacilli and after with the diet influence we have reduction of bifidobacteria, and increase of bacteroids). Artificial breastfeeding is associated with a greater number of clostridium difficile, E. coli and B. fragilis and unless Lactobacilli. The composition of the gut microbiota stabilizes towards 3 years of life. In adulthood, its composition is relatively stable, but it undergoes several chaotic shifts during life (Tab3) [28, 61, 64-66].
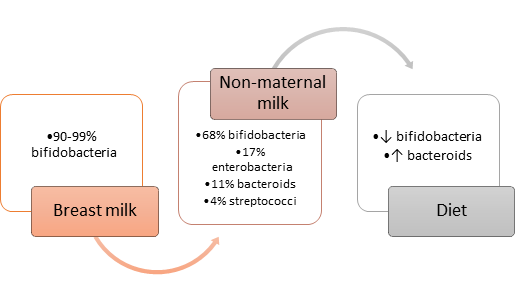
Table 3: Development of the gut microbiota after birth in connection with the first feeding
The microbiota density and composition changes along the gut. Properties of the small intestine, such as short time of transit, high levels of acids, oxygen and antimicrobials limit bacterial growth and allows only that of rapid facultative anaerobes which are also able to adhere and survive on epithelia/mucus, such as Lactobacillaceae. [26, 67-69] In contrast, colonic conditions allow a different community of bacteria’s growth, mainly anaerobes with the ability to utilise complex carbohydrates which are undigested in the small intestine, such as Prevotellaceae, Lachnospiraceae and Rikenellaceae [12, 52, 54, 70]. For what concerns its composition and structure, metagenomic sequencing has identified more than 1000 species of bacteria capable of surviving in the gut, with each of us thought to harbour 160 or so different species. The synonymous ‘microbiome’ is the term used to define the gut microbiota, to better underling its complex genetic mix, derived from the great variety of bacterial species between different individuals. [12, 28, 69, 71, 72] The peculiar ecosystem’s composition possibly depends on different environmental factors such as geographical location, surgery, smoking, depression and living arrangements (urban or rural) but also life events such as illnesses, xenobiotics (toxic substances), drugs treatments (PPLs, H2 Blockers, Prokinetics, Antibiotics, Laxatives, Opioids, NSAIDs), dietary fibre scarcity and possibly also by host genetics leaving room for expansion of pathogenic populations with inter-individual variation. [12, 73-80].
Conclusions
It is not surprising to observe a great attention in the course of history in maintaining good gastrointestinal functions and a normal bacterial flora composition considering the very close relationship existing between the gut microbiota and the host. The application of metagenomics approaches has greatly advanced our understanding of the mechanisms linking the gut microbiota composition and its activity to health and diseases, but many evidences show us a surprising attention of ancient civilizations to preserve its integrity to avoid several acute e chronic gastrointestinal and systemic diseases. A better understanding of its regulating mechanisms will help develop complementary and/or alternative strategies required for maintaining human health.
References
