Antimicrobial and Antioxidant Screening of The Solvent Extracts of The Leaves and Stem of Sesuvium Portulacastrum
Alshrari A.S.,1*, Naira Nayeen2, Alreshidi M.A.3, Mohd. Imran2
|
|
|
Abstract
Introduction: Herbal drugs are an important source of antimicrobials and antioxidants. Purpose: This work was designed to carry out the antimicrobial and antioxidant properties of the solvent extracts of the leaves and stem of Sesuvium portulacastrum grown in Saudi Arabia. Materials and Methods: The leaves and stem of the plant were extracted with methanol and chloroform. The antimicrobial property of the methanolic and chloroform extracts was evaluated by the cup plate method, whereas the DPPH method was used for the evaluation of the antioxidant activity. Results: The methanolic extract of the leaves was a more effective antimicrobial product than the chloroform extract against all the tested bacterial strains, namely, E. coli, S. aureus, P. vulgaris, and K. pneumoniae. The methanolic extract of the leaves also showed better antioxidant activity than the methanolic extract of the stem. Its associated antioxidant effect increases the antimicrobial activity of methanolic extract of the leaves. Conclusion: The leaves of Sesuvium portulacastrum have been identified as a useful source to procure natural antimicrobial and antioxidant agents in human health. Therefore, detailed chemical and molecular level investigations are recommended on the leaves of Sesuvium portulacastrum grown in Saudi Arabia.
Keywords: Sesuvium portulacastrum, Leaves, Stem, Extract, Antimicrobial, Antioxidant.
Introduction
Complementary and alternative medicine refers to diverse medical and health care systems, products, and practices [1, 2], which are not part of the usual medications and treatments [3, 4]. In the recent past, there is a surge in the research of medicinal herbs due to their various biological activities. Herbs could be one of the essential sources to obtain a large number of drugs. Some review articles regarding the antimicrobial effects of herbs have been published [5, 6], which also provide information about useful antimicrobial herbal products. Similarly, reports are available related to the antioxidant effect of the herbal products [7-9]. It has also been reported that the antimicrobial activity of a plant product is increased in the presence of antioxidants [10]. Accordingly, it is worthful to identify natural herbs possessing dual effects of antimicrobial activity and antioxidant activity.
Sesuvium portulacastrum belongs to the family Aizoaceae and is used by traditional healers for the treatment of various disorders [11, 12]. It is a prostrate perennial herb with thick, branched stems. Roots are present at the nodes. The leaves are sessile, opposite, and green in color while the flower is green on the outer side and pink to reddish on the inner surface and hermaphrodite. The fruit is an inconspicuous capsule. The morphology, description, and the habitat of Sesuvium portulacastrum of Saudi origin is also reported [13]. In our study, we have attempted to evaluate some of the physico-chemical properties, antimicrobial and antioxidant activities of the leaf and stem extract using methanol and chloroform as solvents.
Materials and Methods
Collection and extraction
Sesuvium verrucosum was collected from the outskirts of Rafha, Northern Border Province, Saudi Arabia. It was authenticated by Dr. Naira Nayeem (Faculty of Pharmacy, Northern Border University, Rafha).
Preparation of the extracts
The leaves and stems were separated, washed, and dried in the shade for four days. The dried leaves and stems were pulverized to get a coarse powder. The leaf powder (100 g) and stem powder (100 g) were extracted separately at room temperature using 250 ml of methanol and chloroform for about 36 hours and filtered. The filtrates were evaporated and dried using a vacuum evaporator.
Determination of ash value and extractive value
The extracts were screened for their phytoconstituents as per the standard protocol [14]. The leaves and stem were evaluated for the physicochemical properties such as ash content and extractive values [15]. All chemicals (analytical grade) required for extraction, antimicrobial activity, and antioxidant activity were obtained from Merck and Sigma.
Antimicrobial activity evaluation
Cup plate method was used to evaluate the antimicrobial activity using Staphylococcus aureus (Gram +ve), Bacillus subtilis (Gram +ve), Escherichia coli (Gram –ve), Klebsiella pneumoniae (Gram –ve), and Candida albicans (fungi) [16, 17].
Preparation and Standardization of Stock cultures
The cultures were subcultured in nutrient agar plates and stored in slants as stock cultures. For the evaluation, by inoculating each culture from slants to the flask in sterile plates, the stock culture was prepared and incubated at 370C for 24-30 hours. The prepared stock culture was serially diluted as per the requirement and was spread over plates and incubated. The colony-forming units (CFU) were counted from the plates of the various dilutions followed by the calculation of the total CFU in the stock culture. The stock cultures of 1x105 CFU per ml were used for antimicrobial screening.
Determination of the antimicrobial activity
The cup plate method was used to evaluate antimicrobial properties. The concentration used was 200 and 100 μg/ml against all the microorganisms. After preparing the sterile nutrient agar plates, 0.1 ml of the inoculum was taken from the standardized culture of the organism and was spread uniformly. A sterile borer (10 mm diameter) was used to prepare the wells. The solution of the extracts and the standard antibiotics were added to each well separately and tested against the microorganisms. Streptomycin (100 μg/ml) and Fluconazole (25 μg/ml) were used as standards. An optimal temperature was used to incubate the plate for 24 hours at 37oC. This was followed by the measurement of the zone of inhibition (mm) around the well.
Determination of the antioxidant activity
The extracts were evaluated for their antioxidant activity by using 1,1-Diphenyl-2-picryl-hydrazyl (DPPH). The stock solution of the extracts were prepared (10 mg/ml) in methanol [18]. The stock solution of the extracts was utilized to make the working solutions in the following dilutions 20, 40, 60, 80,100, and 120 μg/ml. A 0.002% solution of DPPH was prepared in methanol, and 1 ml was added to the standard and the samples. Ascorbic acid was used as a standard for comparison. Measurements were taken in triplicate. The prepared solutions were then kept in the dark for about 30 minutes, followed by the analysis of the absorbance at 517 nm. A mixture (1:1, methanol: DPPH) was used as a blank. The % absorbance was calculated using the formula.
% Absorbance=A-B2 x 100
Wherein, A = absorbance of the blank, and B = absorbance of the sample.
Results and Discussion
The evaluation of the physicochemical parameters like ash values and extractive values gives an insight into the authenticity, quality, and purity of the drugs. It is essential for standardization for natural medicines [19]. The extractive values are helpful in the detection of contamination and also assist in the evaluation of the solubility of drugs [20]. The ash values and the extractive values are as depicted in Table 1.
Table 1: Ash values and the extractive values of Sesuvium verrucosum
|
Parameter |
Type |
Leaf |
Stem |
|
Ash values (% w/w) |
Total Ash |
1.92 |
1.1 |
|
|
Acid insoluble ash |
1.18 |
0.61 |
|
|
Water-soluble ash |
0.80 |
0.19 |
|
Extractive valves (% w/w) |
Methanol |
12.6 |
7.4 |
|
|
Chloroform |
4.9 |
3.1 |
The amount of the total ash, acid-soluble, and the water-soluble ash in the leaves was found to be 1.92%, 1.18%, and 0.80%, respectively. The extractive content was higher in methanol when compared to chloroform.
Many plants possess antioxidant and antimicrobial activity because of the presence of various phytochemicals such as phenolic acids, tannins, flavonoids, etc. [21, 22]. These natural constituents are present in plant parts such as wood, bark, leaves, roots, etc. Phenolic compounds exhibit a wide range of biological activity, some of them being anti-allergenic, antioxidant, anti-inflammatory, antimicrobial, cardioprotective, and vasodilatory. Plant phytoconstituents that have phenolic hydroxyl groups might be the cause of inhibition of hydrolytic enzyme or other interactions for the inactivation of microbial adhesions. Similarly, the various bacteria may exhibit different degrees of sensitivity to multiple antimicrobial compounds like ketones, aldehydes, phenolic compounds, and esters. Preliminary phytochemical analysis was done on the leaves and stem extracts of the plant that were taken into consideration. Glycosides, phenolic compounds, flavonoids, carbohydrates, sterols, tannins, and proteins were detected in the methanolic extracts, while the chloroform extracts contained flavonoids and steroids. The results of the planned activities varied for the plant part and the solvent used. The literature reports that the type and quantity of the phytoconstituents may be dependent on the part of the plant and the solvent used for extraction. This, in turn, affects biological activity [23-25].
The cup plate method was used for antimicrobial activity, whose results are as shown in Table 2.
Table 2: Antimicrobial activity of the methanolic (ME) and chloroform (CH) extracts of leaves and stem of Sesuvium verrucosum at 200 μg/ml
|
Extract |
Solvent |
E. coli |
P. vulgaris |
S. aureus |
K. pneumoniae |
C. albicans |
|
Leaves |
ME |
11.16±0.82* |
9.20±0.55* |
9.86±0.53* |
4.20±0.14* |
NI |
|
|
CH |
10.3±0.33* |
7.36±0.14* |
7.7±0.27* |
2.26±0.28* |
NI |
|
Stem |
ME |
8.36±0.14* |
8.13±0.21* |
7.1±0.09* |
NI |
NI |
|
|
CH |
7.46±0.23* |
5.43±0.20* |
6.3±0.09* |
NI |
NI |
|
Standard drugs |
Streptomycin |
22.16±0.19* |
25.3±0.18* |
27.1±0.24* |
17.7±0.29* |
- |
|
|
Fluconazole |
- |
- |
- |
- |
12.4±0.92* |
*p < 0.05; NI = No inhibition; Values are expressed as Mean±SD.
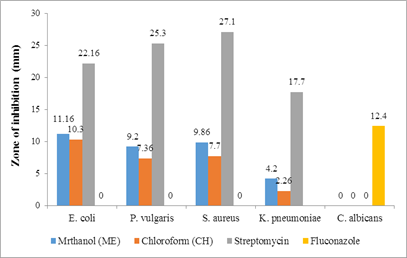
Figure 1: Antimicrobial activity of the leaves extract
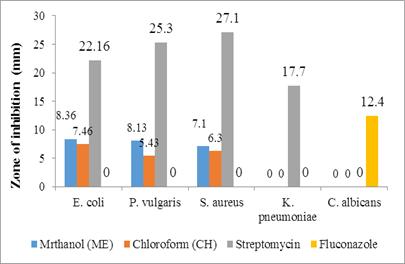
Figure 2: Antimicrobial activity of the stem extract
The plant extracts at 200 μg/ml exhibited varying degrees of antimicrobial activity against the microbes, as shown in Table 2 (Figure 1 & Figure 2). Methanolic extracts showed superior activity when compared to chloroform extracts. The methanol extract of the leaves exhibited more action against the bacterial strains, while the stem extract did not show any activity against K. pneumoniae. None of the experimental extracts demonstrated antifungal activity against C. albicans. During any study, it would be expected that most of the extracts would be active against Gram-positive compared to Gram-negative bacteria [26]. However, in our research, the plant extracts of the leaves and stem were effective against both Gram-positive and Gram-negative bacteria. This can be attributed to the presence of a broad spectrum of antibiotic content in these extracts.
There are several methods for the evaluation of the antioxidant activity of the plant extracts. However, the most common method used is the DPPH method. This method is easy to perform, rapid, and sensitive. The results of the antioxidant activity are depicted in Table 3.
Table 3: Antioxidant activity of the methanolic (ME) and chloroform (CH) extracts of Sesuvium verrucosum
|
Extract / Ascorbic acid |
%antioxidant activity at different concentrations (μg/ml) |
IC50 (μg/ml) |
||||||
|
10 |
20 |
40 |
60 |
80 |
100 |
120 |
||
|
LME |
45.98 |
86.60 |
89.09 |
90.65 |
91.56 |
92.21 |
93.76 |
10.332 |
|
LCH |
42.92 |
74.14 |
75.70 |
77.57 |
78.81 |
80.68 |
82.86 |
10.944 |
|
SME |
40.06 |
81.30 |
81.61 |
83.48 |
86.91 |
88.47 |
90.34 |
10.931 |
|
SCH |
31.33 |
74.76 |
76.32 |
78.13 |
79.75 |
81.30 |
82.24 |
11.842 |
|
Ascorbic acid |
48.78 |
90.34 |
92.18 |
92.83 |
93.76 |
95.32 |
95.95 |
10.088 |
LME = Leaf methanol extract; SME = Stem methanolic extract; LCH = Leaf chloroform extract; SCH = Stem chloroform extract.
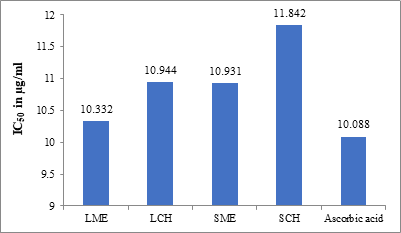
Figure 3: Plot of the antioxidant activity (IC50 in µg/ml) of LME, LCH, SME, SCH, and ascorbic acid
The phenolic compounds present in the plants play a significant role in antioxidant activity. They may exert their activity through various mechanisms. Some of them are scavenging the reactive species, suppression of the lipid peroxidation recycling, binding to the pro-oxidant metals, or increase the activity of antioxidant enzymes. The antioxidant activity of the plant can be due to its redox properties, which allows it to act as a free radical scavenger [27]. The antioxidant activity results revealed that the methanolic extract of the leaves (IC50 = 10.332 µg/ml) showed better activity when compared to the methanolic extract of the stem (IC50 = 10.931 µg/ml). The chloroform extracts of the stem (IC50 = 11.842 µg/ml) and leaves (IC50 = 10.944 µg/ml). Several researchers have reported the variation in antioxidant activity of different parts (leaves, stem, flowers, barks, and roots) and the whole plant [28-30]. The different solvent systems used to extract the samples are the probable reason for the difference in the results. These results reveal that the methanolic extract shows slightly higher radical scavenging activity, which is because of its stronger proton-donating abilities.
The methanolic extracts of the leaves and stem have exhibited significant antimicrobial and antioxidant activity. This was attributed to the presence of phytoconstituents such as terpenoids, essential oils, flavonoids, sterols, and phenolic compounds. Capsaicin, gallic acid, benzoic acid, and epicatechin have also been reported to be present in this plant, which possesses notable antioxidant and antibacterial properties [31, 32]. In a report, it was revealed that antimicrobial activity is increased in the presence of antioxidants [10]. This could be due to their synergistic action. This study could help identify better and newer sources of natural antimicrobial and antioxidants and use them in food and pharmaceutical industries.
Conclusion
Our study results demonstrate that various parts of the plant exhibited variations in their antioxidant and antimicrobial effects, depending on the part and the solvent used. Methanolic extracts of the leaves and stem showed higher antioxidant and antimicrobial activity. Therefore, the plant species must demonstrate these activities to be further subjected to investigation to isolate, identify, and characterize the phytoconstituents for pharmacological testing. From the results of our study, we may conclude that Sesuvium portulacastrum could be considered as a useful source to procure natural antioxidative, antimicrobial agents for use in human health.
Acknowledgement
The authors are thankful to the Northern Border University for providing facilities to perform this research work.
Conflict of Interest
All authors declare that there is no conflict of interest.
Author Contribution
Alshrari A.S., Naira Nayeen, and Alreshidi M.A., conceived the idea and carried out the experiments. Alshrari A.S., Naira Nayeen, and Alreshidi M.A. and Mohd. Imran analyzed the results. Naira Nayeen, Alreshidi M.A.and Mohd. Imran, drafted the manuscript. ASA reviewed the manuscript. All the authors approved the manuscript.
References
