Antitumor Activity of Alkaloids Extract from Opuntia Polyacantha Plant Using High Content Screening Technique (HCS)
Abtisam F. Al-Shukri 1, Farah A. Al-Marzook 2, Nawal A. Al-Hammadi 3 Iman H. Mutlag 4
|
|
|
Abstract
Background/aim: Multipara metric toxicity analyses at the individual cell level using cellular imaging-based methods such as High Content Screening (HCS) play a crucial role in detecting toxicity. The positive result of the toxicity on cancer cell increase with increasing concentration. This study aimed to assess the potential effect of alkaloid extract on breast cancer cells. Material and Methods: Opuntia polyacantha leaves were separately extracted with 80% methanol, and chloroform at pH 2 and 10, and the chloroform portion was dried to obtain the total alkaloid extract. The total alkaloid was qualitatively detected by Hager’s, Dragendorff’s, and Mayer’s reagents. The cytotoxic effect of Opuntia polyacantha alkaloids extract was examined in one-cultured cellular models (MCF7 breast cancer cell line) by HCS. The inhibitory effect of Opuntia polyacantha on MCF7 cell growth was due to the induction of apoptosis. Result: The study found that alkaloid extract of Opuntia polyacantha has the ability to reduce the viability of breast cancer cells by nuclear condensation, cell membrane permeability, disrupting Mitochondrial Membrane Potential (MMP), fragmentation and releasing cytochrome c from the mitochondria into the cytosol. Thus, Opuntia polyacantha is a potential MCF7 inhibitor compared to doxorubicin as the positive control. Conclusion: In this study, data showed that Opuntia polyacantha might have therapeutic value in breast cancer treatment worthy of further attention.
Keywords: Opuntia polyacantha, alkaloids extract, MCF-7, HCS
Introduction
Cancer, as a malignant neoplasm, is a disease in which a group of cells shows abnormal proliferation, invasion, and sometimes metastasis [1, 2]. It remains a major global health burden and is currently ranked as the 3rd leading cause of death worldwide after cardiovascular and infectious diseases [3, 4]. Cancer is the result of cells growing out of control in a part of the body [5]. Secondary metabolites are natural products produced by different sources like plants, microorganisms, and others [6]. These compounds are organic substances having a relatively small molecular weight (<3,000 Daltons) and a considerable structural diversity. These compounds tend to be in a chiral form exhibiting biological activity and facilitate species survival by attracting or repelling other organisms [7]. Plants are among the sources of natural products targeted in the search as potential natural drug candidates. This led to the discovery that plants are not only a source of potential drugs but also an important tool for therapeutics, as they are the source of most novel compounds used by today’s pharmaceutical industries [8]. It is estimated that >80% of pharmaceutical and therapeutic essential compounds are derived from natural products [9].
Material and Methods
The plant parts were washed with tap water to remove dust and then with distilled water (DW) and were shade-dried at room temperature for 10 days. Each dried part was ground and stored in an airtight container to prevent the humidity effect and then stored at room temperature until further use. The total alkaloid was extracted according to Harborne [10]. In summary, 20g of the dry powder of plant was extracted with 80% methanol for 24h in a continuous extraction using a 250-ml Soxhlet apparatus. The extract was then filtered by Whatman No.1 filter paper and concentrated in a rotary evaporator at 45°C under vacuum until it reached to 10ml. After that, it was poured into a separating funnel and 2N HCl was gradually added to adjust the pH up to 2, Consequently, the extract was washed with 10ml chloroform 3 times, and the pH was set to 10 using NH4OH, and partitioned with 10ml chloroform 3 times. The chloroform portion was dried to obtain the total alkaloid extract.
Multiple Cytotoxicity Assay (HCS):
Cellomics multi-parameter Cytotoxicity 3 Kit was utilized for HCS. This kit measures 6 independent parameters simultaneously in the same cell including changes in cell membrane permeability, cytochrome c release, MMP changes, morphological and nuclear changes, cell loss, and cell health. Briefly, 24h after AuL2 treatment, the cell permeability dye and MMP dye were added to live cells and incubated at 37°C for 30min. Cells were fixed, permeabilized, and blocked with blocking buffer (1X), then they were probed with primary cytochrome c, primary antibody, and secondary DayLight 649-conjugated goat anti-mouse IgG each for 1h. The nucleus was stained with Hoechst 33342. ArrayScan HCS system (Cellomics, PA, USA) was used to analyze plates containing stained cells. This system is an automated computerized fluorescence imaging microscope that automatically detects stained cells and reports the distribution and intensity of fluorescence in individual cells. 1000 cells were analyzed in each well. Images were taken for each fluorescence channel using proper filters. The average fluorescence of the cell population, and also images and data about the texture and intensity of the fluorescence within each cell, were saved in Microsoft SQL database for easy retrieval. Data Viewer version 3.0 (Cellomics) and Array Scan II Data Acquisition were used to capture, extract, and analyze data [11].
Statistical Analysis
The data were statistically analyzed using the SPSS 14.0 version using the one-way ANOVA according to the method described by Levesque [12] numerical data were expressed as mean ± standard deviation. p<0.05 was considered statistically significant.
Result
Results in figure (1) show that the viability of MCF-7 cells decreased by increasing the concentration of alkaloids extract as compared to untreated cells. When treated with a high concentration (400 µg/ml), the reduction in cell counts was (2213) while it was (3840) in the untreated cells. There was no significant difference between high concentration (400 μg/ml) and positive control (Doxorubicin 20 μm; Doxorubicin is a chemotherapy medication used to treat acute lymphocytic leukemia, lymphoma, Kaposi's sarcoma, bladder cancer, and breast cancer [13].
Figure (2) shows images taken from a multi-parametric (cytochrome C releasing, mitochondrial membrane potential, cell membrane permeability, and nuclear intensity). The results showed that MCF-7 nuclear intensity increases significantly when treated with 400 μg/ml of the fraction 525.3±62.90 (figure 3). The exposure of MCF-7 cells to higher concentrations 400 μg/ml of fraction showed remarkable changes in nuclear morphology and size when compared with the positive control (Doxorubicin 20 μm), and negative control (untreated cell) resulted in cell loss and caused increased in nuclear size due to nuclear swelling and increasing the cell membrane permeability. The morphology of the nucleus (Hoechst blue) showed a nuclear condensation, which mostly appeared at higher concentrations (400 µg/ml) and was significantly different from that of the untreated cells (figure 2).
As shown in figure (4), the MCF-7 cell membrane permeability (green) was increased gradually in a dose-dependent manner and the significant effect was observed after exposure to 200 and 400µg/ml that was significant when compared with untreated cells(42.18 and 42.18). It has also revealed that the effect of the purified fraction was dose-dependent.
The other two parameters, cytochrome C release and mitochondrial membrane potential were measured. As shown in figure (5), only higher concentrations of the fraction (400 and 200µg/ml) induced a significant reduction in mitochondrial membrane potential which were 210.4 and 272 (RFU), respectively, while the positive doxorubicin control was 225.9 (RFU). The measurement of mitochondrial membrane potential depends on the mean intensity of MMP dye that punctuates the mitochondria within the per-nuclear cytoplasmic region [14] and the lower the fluorescent intensity, the greater the effect against the mitochondria. Furthermore, the release of cytochrome C was only induced significantly after the MCF-7 cells exposed to 400µg/ml of the extract (figure 6).
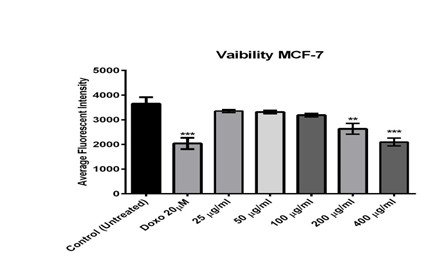
Figure 1: The effect of different concentrations of Alkaloid extracted from Opuntia polyacantha on MCF-7 cells after 24 hours of incubation at 37 ºC, evaluated on the ArrayScan HCS Reader.
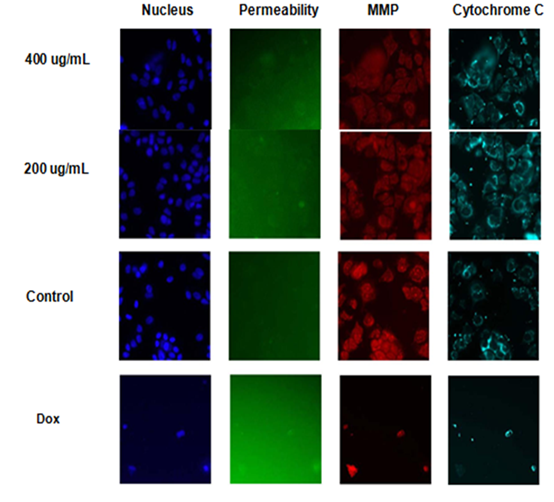
Figure 2: Multiparameter cytotoxicity (HCS) analysis of Opuntia polyacantha (Alkaloids extract) treated with MCF-7 cell line after 24h of incubation at 37ºC. Cells were stained with Hoechst 33342 (Blue)(Ex 330 nm/Em 420nm) Dye, which allows observing the DNA content, nuclear morphology changes, and cell loss Permeability Dye (Green) (Ex 491nm /Em 509 nm) for membrane permeability monitoring, MMP Dye (Red) (Ex552 nm /Em 576 nm) for mitochondrial membrane potential changes, and with goat anti-mouse secondary antibody conjugated with DyLightTMfor Cytochrome C releasing
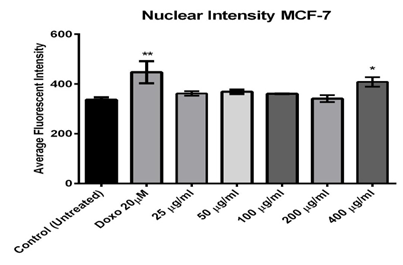
Figure 3: Effect of different concentrations of Alkaloids extracted from Opuntia polyacantha on nucleus size and morphology change using MCF-7 cells after 24 hours of incubation at 37 ºC and evaluated on the ArrayScan HCS Reader.
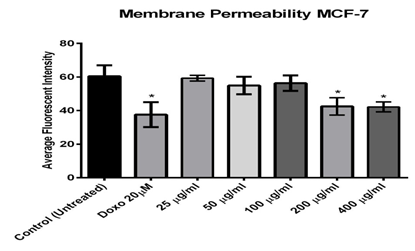
Figure 4: Effect of different concentrations of Alkaloids extracted from Opuntia polyacantha on cell permeability using MCF-7 cells after 24 hours of incubation at 37 ºC and evaluated on the ArrayScan HCS Reade.
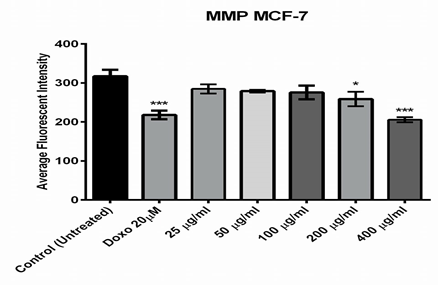
Figure 5: Effect of different concentrations of Alkaloids extracted from Opuntia polyacantha on Mitochondria membrane potential changes using MCF-7 cells after 24 hours of incubation at 37 ºC. and evaluated on the ArrayScan HCS Reader.
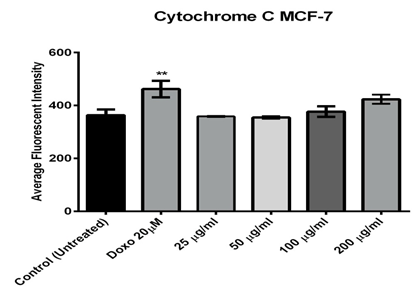
Figure 6: Effect of different concentrations of Alkaloids extracted from Opuntia polyacantha on cytochrome C releasing using MCF-7 cells after 24 hours of incubation at 37º C and evaluated on the ArrayScan HCS Reader
Discussion
Medicinal plants contain some organic compounds including flavonoids, steroids, terpenoids, carbohydrates, alkaloids, and tannins, which provide definite physiological action on the human body [15]. ‘Blocking agents’ are compounds that inhibit cancer initiation. Bioactive components of plants can prevent carcinogenesis by providing alternative targets for electrophonic metabolites, increasing detoxification, or blocking metabolic activation [16, 17]. These compounds act as strong antitumors that intercalate DNA molecules and cause massive DNA damage [18].
The condensed and bright intensity of Hoechst blue stain was contributed to the nuclear condensation and such observations are the typical features of apoptotic cell morphology: nuclear fragmentation, nuclear condensation, cell shrinkage, and formation and aggregation of apoptotic bodies [19, 20] Besides, using the membrane permeability dye and increasing its intensity especially at the highest exposure concentrations supports the fact that the extract could induce apoptosis of MCF-7 cells since it can only stain the cells when plasma membrane permeability increases due to the loss of plasma membrane integrity [21]. It has been reported that changes in cell membrane permeability are often associated with toxic or apoptotic responses, and the loss of cell membrane integrity is a common phenotypic feature of marked cytotoxicity [22, 23]. Many investigations studied the suppressing and cytotoxic activity of plant natural products on tumor cells revealed that these cells showed an increased expression of p53 protein by triggering the up-regulation of the p53 gene and down-regulation of Bcl-2 anti-apoptotic gene [24, 25].
One of the most distinctive features of cell death or apoptosis is the disrupting of active mitochondria [26]. The MMP dye was used to detect the active mitochondria functionality; it can accumulate in mitochondria and preserve the potential of their inner membrane [27]. Compared with the doxorubicin exposure, the results indicated that 400 and 200µg/ml of fraction significantly reduced the mitochondrial brightness, which might be due to the changes of mitochondrial transmembrane potential and triggering apoptosis of MCF-7 cells. Changes in mitochondria play a key role in apoptosis. Mitochondria can proliferate during apoptosis stimulation and lead to increased total mitochondrial mass. The mitochondrial membrane potential dye accumulates in healthy mitochondria, caused by its transmembrane potential, and is absent in depolarized mitochondria that result from a cytotoxic compound [28]. It was suggested that changes in the membrane potential are due to the opening of the mitochondrial permeability transition pore, allowing the transition of ions and small molecules like calcium ions and in consequence, this leads to a decoupling of the respiratory chain and then, releasing cytochrome C into the cytosol [29]. Finally, the release of cytochrome C activates a series of caspases, cysteine proteases, which are mainly responsible for the degradation and digestion of the cell from inside [30].
Conclusion
Depending on the HCS technique, alkaloids from Opuntia polyacantha appeared cytotoxic activity against cancer cell lines by the effect on (changes in cell membrane permeability, cytochrome c release, MMP changes, nuclear and morphological changes, and cell viability).
References
