Characterization of Promoter Hypermethylation of Tumor Suppressor Gene Rassf1a and Its Association with the Risk of Nasopharyngeal Carcinoma
Hue Hong Thieu1, Thuan Duc Lao2, Thuy Ai Huyen Le2*
|
|
|
Abstract
Background: The inactivation of RASSF1A, which caused by promoter hypermethylation, has been reported to be the etiological factor of nasopharyngeal carcinoma in previous studies. However, there was no study carried out to evaluate the status of RASSF1A in Vietnamese NPC patients. Aims: This study was designed to identify and evaluate the hypermethylation frequency of the RASSF1A promoter in nasopharyngeal biopsies as well as the association between hypermethylation of RASSF1A and nasopharyngeal tumorigenesis from Vietnamese NPC patients. Materials and methods: Seventy NPC biopsy samples and seventy non-cancerous swab specimens were collected from the local hospital and analyzed by the Nested-MSP method. Results: The frequency of RASSF1A gene hypermethylation was markedly higher in NPC biopsy samples, counting for 67.14%, compared to non-cancerous samples, counting for 12.86%. A trend toward positive association was found between hypermethylation of the RASSF1A gene and nasopharyngeal carcinoma. The hypermethylation of RASSF1A was significantly associated with an approximately 13.85 fold increase in NPC compared to non-cancerous samples. Moreover, hypermethylation of RASSF1A was found to be correlated with the clinical stage and pathological classification. Conclusion: Our data suggested that the hypermethylation of the RASSF1A gene promoter is significant in nasopharyngeal carcinoma in Vietnamese patients. The hypermethylation of RASSF1A may be a potential biomarker for NPC in the Vietnamese population.
Keywords: RASSF1A, nasopharyngeal carcinoma, hypermethylation.
Introduction
Cancer became a major issue with increased carcinogenic exposure [1]. Prostatitis induced prostate carcinoma, chronic pancreatitis induced pancreatic cancer, Hepatitis B induced hepatocellular carcinoma, HPV induced cervical cancer, and pharyngeal cancer can be named as some inflammatory conditions or injuries that are related with malignancy [2]. The silencing of TSG (tumor suppressor genes), caused by the phenomenon of hypermethylation - an event of epigenetics alterations, has been reported as one of three etiological factors of nasopharyngeal carcinoma [3-5]. Many TSGs have been recorded to be involved in the critical cancer-related cellular pathways. Thus, the patterns of TSG promoters’ methylation have been studied and reported to be acted as the potential epigenetic biomarkers for NPC [4, 6-8]. Among them, RASSF1A (RAS-association domain family 1 isoform A), located 3p21.31, belonged to the family of RAS effectors, which is a tumor suppressor gene. It functioned as the protein that modulates multiple apoptotic, cell cycle pathways, and DNA repair process [7-9]. In a study of mouse and human lung epithelial cells in Germany, DNA damage was found along with increasing OH group in these cells [10]. Increasing pieces of evidence demonstrated that the inactivation of RASSF1A due to hypermethylation has been reported to associate with the pathogenesis of NPC [7-9, 11]. The hypermethylation of RASSF1A in NPC has been reported in many populations in the world. For example, Lo et al. reported that the frequency of RASSF1A promoter methylation in NPC primary tumor was 66.67%, and not in the normal nasopharyngeal epithelial sample [4]. In the pollution of Tunisia, a country located in Africa, Challouf et al reported that the rate of RASSF1A promoter methylation in NPC biopsy samples was 75%, and no methylation was recorded in non-cancerous samples [12]. However, there are still some inconsistencies in the report of frequency of RASSF1A gene promoter hypermethylation, due to the source of samples, population, etc. Giving examples, in the study of Chang et al, the frequency of RASSF1A promoter hypermethylation in NPC patients was 66.7%, 3.3%, and 33.3% in biopsy tissue, blood samples, and brushing samples, respectively, in Hongkong population [13]. Another study performed on the population of Hongkong, Tian et al. reported the low frequency of RASSF1A in the NPC serum sample was 17.5% in the Hongkong population [14]. Therefore, it could be inferred that the frequencies of hypermethylated RASSF1SA in different populations as well as the source of samples may be different. The use of the different sources of samples may collect the evidence the methylation of TSGs and could be used as the target genes to develop the therapeutic approach in NPC [15]. Vietnam is well known as the high incidence and mortality rate of nasopharyngeal carcinoma. However, the study involved in the methylation of tumor suppressor genes, including RASSF1A, was not well performed. Therefore, this first case-control study was aimed to assess the relationship between RASSF1A promoter methylation in NPC tumor biopsy samples and NPC risk in the Vietnamese population.
Materials and Methods
Ethics statement, sample collection
The Institutional Ethics Board approval was obtained from the Medical Ethics Committee of the Cho Ray Hospital, Ho Chi Minh City, Vietnam (The decision number of the permission: 516/BVCR-HDDD). All samples, enrolled in the current study, were obtained from all participants, who were required to sign the consent forms to approve the usage of the samples for laboratory work and analysis.
A total of 70 NPC biopsy tissues and 70 non-cancerous samples that were archived from the local hospital, Vietnam. All the biopsy samples were submitted to the histopathological department and were subsequently confirmed by hematoxylin and eosin staining. The non-cancerous nonmalignant swab samples that were negative for NPC were collected from non-NPC patients. In brief, a 15-cm-long cotton stick was inserted into the nasal cavity and moved toward the nasopharyngeal wall; it was then swept over the surface of the posterior and lateral nasopharyngeal wall. The cotton stick was withdrawn and immediately immersed in phosphate-buffered saline stored at -20oC for further experiments.
DNA extraction and bisulfite modification
The total genomic DNA was isolated from biopsy or swab samples by the phenol/chloroform method. Cells obtained from samples were lysed in lysis buffer (10 mM Tris-HCl pH = 8, 10 mM EDTA, 150 mM NaCl, 2% SDS) containing Proteinase K (0.1 mg/ml). Then, the total of genomic DNA was isolated and purified by using standard phenol-chloroform and ethanol precipitation. The bisulfite conversion of 2 μg genomic DNA was performed using
EpiJet Bisulfite Conversion Kit (Thermo Scientific, #1461). The final precipitation was eluted in a volume of 20 μl and stored at -20oC for further studies.
Nested methylation-specific polymerase chain
Nested-MSP assay was performed to characterize the methylation status of the RASSF1A gene promoter in samples. The primers used in stage 1 and stage 2 amplification were listed in Table 1. Each stage of PCR was performed in a total of 15 μl containing 200 ng bisulfite-modified template DNA (in case of stage 1 PCR) or 200 ng stage 1 PCR product (in case of stage 2 PCR), 10 μM each primer, and 7.5 μl MyTaqTM Mix (Bioline, #25041). The thermal cycling was initiated at 95oC for 5 min, followed by 40 cycles of denaturation at 95oC for 30 sec, annealing at the XoC for 30 sec, extension at 72oC for 30sec, and a final extension at 72oC for 10 min (Note: XoC was the specific annealing temperature for each specific methylated or unmethylated primer, XoC = 57oC, 58oC and 60oC for Stage 1, methylated and unmethylated primers, respectively). Finally, the PCR products of the methylated and unmethylated were separated on 2% agarose gel and visualized by ethidium bromide staining. MSP products were sequenced to confirm the specificity of primers, examine the efficiency of bisulfite modification and the hypermethylation status of the target gene.
Statistical analysis
Statistical analyses were performed using Medcalc® Version 12.7.0.0 that used the Chi-square test for sample size. The correlation between methylation status and HPV was examined using the Chi-squared test. The differences in methylation frequencies of RASSF1A among groups were considered statistically significant for p ≤ 0.05. Moreover, the odds ratio (OR) with 95% confidence intervals (CI) were also evaluated.
Results
Characteristic of sample collection
The characteristics of all 70 NPC patients are summarized in Table 1. Among them, the number of males (accounting for 77.14%) is more than that of females (accounting for 22.86%). The mean age of the 70 NPC cases was 52.89 ± 1.67 (range: from 20 to 81). The age incidence profile indicated an increase in NPC risk up to late middle age (range: from 40 to younger than 60), counting for 47.14%. Tumor histological types were classified according to the World Health Organization (WHO) classification for NPC criteria. Type 3 (undifferentiated carcinoma [UC]) accounted for the highest proportion of all types of NPC; 67.14% (n = 47) of NPC cases. 51.43% (n = 36) of NPC patients were diagnosed in stage 4 and no case was recorded in stage 1.
Hypermethylation status of RASSF1A gene promoter
In the current study, the Nested-MSP method was applied to evaluate the hypermethylation status of RASSF1A in both cancerous and non-cancerous samples. The Nested-MSP products of hypermethylation and unmethylation were observed in the band of 200 bps and 173 bps in length by electrophoresis, respectively (Fig. 1). The hypermethylation and unmethylation sequences were confirmed by sequencing. As a result, the methylated Cytosine was unchanged, which were marked by square symbols (Fig. 2A). Otherwise, all the unmethylated Cytosines were changed into Thymine, which was indicated by triangle symbols in sequence (Fig. 2B). Thus, we successfully carried the bisulfite modification and Nested-MSP assay to evaluate the aberrant methylation status of the RASSF1A gene in samples. As a result, the promoter hypermethylation and unmethylation frequencies for RASSF1A in NPC samples were 67.14% (47 of 70 samples), and 32.86% (23 of 70 samples), respectively. In a group of control samples, the hypermethylation and unmethylation frequencies for RASSF1A were 12.86% (9 of 70 samples), and 87.14% (61 of 70 samples), respectively. The p < 0.0001 indicated that the hypermethylation of RASSF1A in the group of cancer samples was significantly higher than in the control group. Also, it was indicated that there was a strong and significant association between the hypermethylation of RASSF1A and nasopharyngeal carcinoma. The OR was 13.85 (95% CI = 2.62 – 10.04, p < 0.0001), which was once again confirmed the significant association between RASSF1A and nasopharyngeal carcinoma.
Clinicopathological significance of RASSF1A promoter hypermethylation
A significant correlation was observed between the frequency of promoter hypermethylation of RASSF1A and pathological classification (p = 0.04), clinical stage (p = 0.03), but no correlation was observed between promoter methylation of RASSF1A and the patients’ gender (p = 0.10), age (p = 0.46) (Table 2).
Discussion
The tumorigenesis of NPC has been reported as the multistage process involving the infection of EBV, environmental factors, and genetic/epigenetic alterations [7, 9, 11]. Recent studies indicated that the inactivation of tumor suppressor genes by epigenetic modification has been considered as the major mechanism for the NPC [4, 6-8]. Among the epigenetic alterations, hypermethylation of the CpG islands of the DNA promoter is the most important and common epigenetic mechanism leading to gene activation [16]. Among tumor suppressor genes, RASSF1A was recently found to be involved the process of nasopharyngeal tumorigenesis, including cell cycle arrest, apoptosis as well as cell proliferation [7-9, 11, 16]. In the study of Dammann et al. 2000, the re-expression of RASSF1A in lung carcinoma cells reduced colony formation suppressed anchorage-independent growth and inhibited tumor formation in nude mice [17]. Concerning NPC, the high frequency of hypermethylation of RASSF1A in NPC clinical samples has been reported in many studies [7-9, 11, 16]. In the study by Fendri et al. (2009), RASSF1A hypermethylation as a marker of accelerated tumourigenesis, whereby the authors reported that the hypermethylation of RASSF1A was observed in an early age of onset of NPC patient [18]. Further evidence supported that the hypermethylation of the RASSF1A promoter may be associated with the development, progression, and metastasis of NPC, and acted as the promising non-invasive biomarker for the diagnosis of NPC [19]. These studies indicated the potential role of the RASSF1A gene as the tumor suppressor gene and may act as the valuable biomarker for NPC. However, the profile of RASSF1A methylation has not been reported in Vietnamese NPC patients. Therefore, this was the first case-control study, which involved the evaluation of the hypermethylation status of RASSF1A, which may provide insight into NPC tumorigenesis in Vietnamese NPC patients. Our nested-MSP showed that the RASSF1A methylation was frequent in NPC, as the RASSF1A gene was subjected to methylation in 67.14% of nasopharyngeal biopsy samples. Also, the low frequency of RASSF1A was found in normal nasopharyngeal swab samples, counting for 12.86%. Compared to the previous study, various frequencies of RASSF1A were reported from 3.33% to 82.14% [7, 9, 13, 20]. There was a similar correlation between the methylation of RASSF1A and the risk of NPC, thus, RASSF1A served as the tumor suppressor gene, and inactivated through the hypermethylation. Additionally, the hypermethylation of RASSF1A was significantly associated with an approximately 13.85 fold increase in NPC than compared to non-cancerous samples (OR = 13.85, 95% CI = 2.62 – 10.04, p < 0.0001). A wide range of non-cancerous diseases originated from the squamous and mesenchymal epithelium occurs in the laryngopharynx [21]. Moreover, hypermethylation of RASSF1A was found to be correlated with the clinical stage and pathological classification, which were corresponded to previous studies [16, 22]. Current pieces of evidence supported that the higher hypermethylation frequency was observed in the advantage of NPC (stage III, stage IV, counting for 55.32% sample within RASSF1A methylation (26 of 47 samples)), and undifferentiated carcinoma (counting for 63.83% sample within RASSF1A methylation (30 of 47 samples)). The associations were not observed in the group of patients’ gender and age. These investigations supported the potential of methylation of RASSF1A could be served as diagnostic purposes in Vietnamese NPC patients. The process of methylation could be reversed as there is no any epigenetic alterations on tumor suppressor genes, including RASSF1A, by the employment of the demethylated agent 5-aza- 2'-deoxycytidine. Therefore this suggested that re-expression of RASSF1A may open up the new strategy of NPC therapy.
Conclusion
In summary, this is the first case-control study involving the evaluation of hypermethylation status on RASSF1A – a tumor suppressor gene. The frequency of RASSF1A gene hypermethylation was markedly higher in NPC biopsy samples, counting for 67.14%, compared to non-cancerous samples, counting for 12.86%. Additionally, the significant correlation between RASSF1A hypermethylation and nasopharyngeal tumorigenesis, as well as the odds ratio, calculated as 13.85, was reported in the significant correlation. In a word, the potential biomarker, which based on the detection of RASSF1A promoter hypermethylation, will be an auspicious characteristic for NPC in Vietnamese NPC patients. In a further study, the present findings require an extension to numbers of many sources of a sample to find out the potential non-invasive tumor markers for screening and early diagnosis purposes in Vietnam.
Acknowledgments
We wish to express our thanks to the research project sponsored by Ho Chi Minh City Open University. We thank all the recruited participants in this work and Dr. Nguyen Trong Minh, Dr. Nguyen Huu Dung, and all the staff members of Otorhinolaryngology in Cho Ray hospital, Ho Chi Minh City, for collecting the samples used in these studies.
Conflict of interest
The authors declared that they have no competing interests.
References
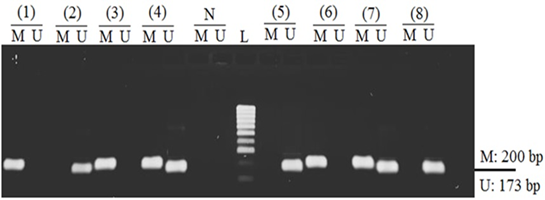
Figure 1. The status of hypermethylation, and unmethylation of RASSF1A gene promoter in representative samples. M: Hypermethylation; U: Unmethylation; L: Molecular weight ladder 100 bps.
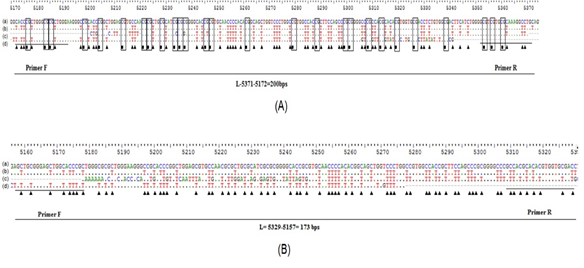
Figure 2. Sequencing profile of (A) hypermethylation, and (B) unmethylation of RASSF1A gene. (a) Sequence without bisulfite modification, (b) Sequence without bisulfite modification by Methprimer software; (c)(d) Sequence of Nested-MSP products by forward and reversed primer.
Table 1. Characteristic of NPC tumor biopsy sample
|
|
n (%) |
|
Gender Male Female |
54 (77.14) 16 (22.86) |
|
Age ≤20 20 to ≤ 40 40 to ≤ 60 60 to ≤ 80 >80 |
1 (1.43) 14 (20.00) 33 (47.14) 21 (30.00) 1 (1.43) |
|
Pathological classification Type 1 Type 2 Type 3 |
2 (2.86) 21 (30.00) 47 (67.14) |
|
Stage I II III IV |
0 (0.00) 25 (35.71) 9 (12.86) 36 (51.43) |
Note: Type 1, keratinizing squamous cell carcinoma; type 2, non-keratinizing carcinoma; type 3, undifferentiated carcinoma.
Table 2. Association between RASSF1A promoter methylation and clinicopathological index in NPC
|
|
RASSF1A |
|
|
P (%) |
N (%) |
|
|
Gender |
|
|
|
Male |
33 (47.14) |
21 (30.00) |
|
Female |
14 (20.00) |
2 (2.86) |
|
p |
0.10 |
|
|
Age |
|
|
|
≤20 |
1 (1.43) |
0 (0.00) |
|
20 đến ≤ 40 |
11 (15.71) |
3 (4.29) |
|
40 đến ≤ 60 |
22 (31.43) |
11 (15.71) |
|
60 đến ≤ 80 |
13 (18.57) |
8 (11.43) |
|
>80 |
0 (0.00) |
1 (1.43) |
|
p |
0.46 |
|
|
Pathological classification |
|
|
|
Type 1 |
0 (0.00) |
2 (2.86) |
|
Type 2 |
17 (24.29) |
4 (5.71) |
|
Type 3 |
30 (42.86) |
17 (24.29) |
|
p |
0.04 |
|
|
Clinical stage |
|
|
|
I |
0 (0.00) |
0 (0.00) |
|
II |
21 (30.00) |
4 (5.71) |
|
III |
7 (10.00) |
2 (2.86) |
|
IV |
19 (27.14) |
17 (24.29) |
|
p |
0.03 |
|
