CHEMICAL CONSTITUENTS FROM THE SEEDS OF Alpinia blepharocalyx K. Schum in VIETNAM AND THEIR BIOACTIVES
Ngoc-Tuan Nguyen1, Thi-Huong Nguyen2, Trung-Hieu Tran2, Thanh-Huyen Mai Thi3, Nu-Trinh Nguyen Thi1, Xuan-Hai Tang4, Viet-Hai Ha5, Gia-Buu Tran1, Dinh-Thang Tran3,*
|
|
|
ABSTRACT
Introduction: Today, Alpinia plants are used to treat many diseases such as enterozoa, vomiting, gastralgia, indigestion, etc. in Japan, China, and India. The main second metabolites compounds of Alpinia were terpenoids, steroids, alkaloids, diarylheptanoids, flavanones, etc. As a continued program aiming to search for new metabolites from the Alpinia plants, A. blepharocalyx K. Schum. was collected and studied. Materials & Methods: This study aimed to isolate the bioactive compounds from the seeds of Alpinia blepharocalyx K. Schum (SAB) and evaluate their anti-cancer and anti-inflammatory effects in Vietnam. The compounds of SAB were determined via MS, IR, and NMR spectrum analysis, and their anticancer activity was tested against cancer cell lines (KB, SK-LU-1, MCF7, and HepG2) via the MTT assay. Moreover, the anti-inflammatory effect of the compounds was measured by evaluating the elastase release inhibition and the assays of the superoxide anion generation. Results & Discussion: We identified a sesquiterpene (alpiblepharin A) and eight flavonoids (flavokawain A, 2', 6'-dihydroxy-4'-metoxychalcone, nevadensin, apigenin, apigetrin, cynaroside, rutin, polydatin) from the SAB extract. This compounds showed significant anti-inflammatory (IC50 values of 4.3 ± 0.3 and 6.3 ± 1.7 μM) and cytotoxic activity (IC50 values of 15.23 ± 2.14 - 25.81 ± 2.45 μM/mL) against cancer cell lines (KB, SK-LU-1, MCF7, and HepG2). Conclusion: The data suggested Alpinia blepharocalyx K. Schum. as a promising functional food for the treatment of inflammatory and cancer-related diseases.
Keywords: Alpinia blepharocalyx, alpiblepharin A, sesquiterpene, cytotoxic activity, anti-inflammatory
Introduction
Medicinal plants have widely been used as since the ancient times to treat various diseases [1-4]. The genus Alpinia is an important member of the Zingiberaceae family that includes 230 species [5] and most of them are distributed in Asia, including India, Vietnam, Malaysia, China, and Japan. Today, Alpinia plants are used to treat various diseases such as gastralgia, indigestion, vomiting, etc. in Japan, China, and India. Many groups of substances, including terpenoids, steroids, alkaloids, diarylheptanoids, flavanones, etc. have been isolated from this genus [6]. The plant has been traditionally used to treat skin disorders [7]. A number of diarylheptanoids and phenolic compounds have been isolated from the plants. These compounds are known to exhibit in vitro NO inhibitory, antiproliferative, and cytotoxicity activities [8]. In this study, SAB was collected and studied as a continued program aiming to search for new metabolites of the Alpinia plants. Herein we wish to publish the characterization of alpiblepharin A (1). Moreover, eight known compounds were isolated from this sample. Moreover, the anticancer, anti-inflammatory effects of the compounds were tested and which provided more information to use this product as a potential functional food in the future.
Materials and Methods
Plant Material. The SAB were collected from the Quephong district of Nghean province, Vietnam in September 2018 and was identified by Dr. Nguyen Quoc Binh, Vietnam National Museum of Nature, Hanoi, Vietnam. A voucher specimen (sample code AB092018VN) was deposited at the School of Chemistry, Biology, and Environment, Vinh University.
General Methods. The 1H- and 13C-NMR, HSQC, and HMBC spectra were obtained on the Bruker AV-III 500 NMR spectrometer, with tetramethylsilane (TMS) as the internal standard and chemical shifts were reported in δ values (ppm). The high-resolution electrospray ionization (HR-ESI) mass spectra were measured using an Agilent 1200 LC-MSD Trap spectrometer. The preparative HPLC was conducted on the Agilent 218 purification system using a Shim-pack XR-ODS II column (2.0 × 100 mm, inner diameter 2.2 μm). Sephadex LH-20 (Sigma Aldrich) and 40–60 μm silica gel (Merck) were used for gel and column chromatography. The spots of TLC were visualized at 254 and 365nm. TLC was heated silica gel plates sprayed with Ce(SO4)2 reagent. All solvents used in column chromatography were distilled.
Anti-inflammatory bioactivity. The anti-inflammatory bioactivity was determined by elastase release inhibition examinations and the superoxide anion generation [9, 10].
Cytotoxicity Assay. The cancer cell lines [HepG2 (ATCCHB8065), MCF7 (ATCCHTB-22), SK-LU-1 (ATCCHTB-57), and KB (ATCCCCL-17)] were maintained in Dulbecco′s D-MEM medium [penicillin G (100 UI/mL, L-glutamine (2 mM), 10% fetal calf serum), gentamicin (10 μg/mL), and streptomycin (100 μg/mL)]. Stock solutions were prepared in DMSO-H2O (1/9 v/v), and cytotoxicity assays were performed in 96-well microtiter plates against cancer cell lines (KB, SK-LU-1, MCF7, and HepG2) (3 × 103 cells/mL) using a modification of the published method [11].
Extraction and Isolation. The SAB were dried at 25°C for 48 h. Then, this material (9.0kg) was extracted with methanol (MeOH) by using the mercerization method (3 × 20 L) at room temperature. The solvent extract was evaporated under low pressure at 40-50 °C in order to yield a crude methanol extract (950 g). The crude methanol extract was partitioned by liquid-liquid extraction method with hexane, ethyl acetate, butanol successively. The solvent was recovered under low pressure to afford three crudes including crude hexane (ABH, 20 g), crude ethyl acetate (ABE, 345 g), crude butanol (ABB, 189 g), and crude water (ABW, 126g). The ethyl acetate crude (ABE) was isolated by silica gel column chromatography (CC) with hexane/ethylacetate step gradient system (100/1 – 0/1, v/v) to yield eight fractions (Frs. ABE1-ABE8). Fraction ABE1 (9.8 g) eluted with hexane/acetone (10/1, v/v) was further separated using CC and purified by preparative HPLC to obtain compound 1 (12 mg). Fraction ABE2 (43 g) was subjected to CC eluting with hexane/ethyl acetate solvent mixture (15/1) to give six subfractions (Frs. ABE 2.1-ABE2.6). Purification of fractions ABE2.1 (0.3 g) was performed by using RP-18 eluted with methanol/water (9/1, v/v) to yield compound 2 (35 mg). Purification of fractions ABE2.1 (0.3 g) was subjected to the Sephadex LH-20 eluted with methanol/water (9/1, v:v) to produce compound 4 (22 mg). The fraction ABE5 (29 g) was subjected to CC eluted with chloroform/methanol (20/1, 10/1 v/v) in the order of increasing polarity, to give five subfractions (ABE 5.1-ABE5.5). Purification of fractions ABE5.2 (1.2g) was performed by using RP-18 eluted with methanol/water (3/1, v/v) to produce compound 3 (7 mg). Fraction ABE5.5 (1.5g) was subjected to CC eluting with a solvent mixture of chloroform/methanol (7/1,v/v ) to yield compound 5 (15 mg). The soluble extracts (ABB) were applied to silica gel column chromatography with a chloroform/methanol step gradient system (100/1 – 0/1, v/v) to give seven fractions (Frs. ABB1-ABB7). Fraction ABB1 (0.8g), eluting with chloroform/methanol (9/1, v:v), was purified by RP-18 to give compound 9 (75 mg). Fraction ABB 2 (0.5 g), eluting with methanol/water (1/1, v/v) was purified with Sephadex LH-20 column chromatography to give compounds 8 (15 mg). Fraction ABB4 (1.7 g), eluting with methanol/water (3/1, v/v), was purified with RP-18 CC to yield compounds 6 (28 mg) and 7 (11 mg).
Statistical analysis
All of the determinations were performed in triplicate. The experimental results were expressed as mean ± standard deviation, and the Statgraphics Centurion XVI software was used to conduct statistical analysis. Differences between different treated groups were analyzed using ANOVA and multiple range tests with p<0.05.
Results and Discussions
Chemical constituents of the SAB
To determine the chemical constituents of A. blepharocalyx, we studied the characterization and bioactives of nine compounds (1-9), which were isolated from SAB. The structures of all compounds were characterized by comparison with the literature. Their structures were identified as alpiblepharin A (1), flavokawain A (2) [12], 2', 6'-dihydroxy-4'-metoxychalcone (3) [13], nevadensin (4) [14], apigenin (5) [15], apigetrin (6) [15], cynaroside (7) [16], rutin (8) [17], and polydatin (9) [18].
Compound 1 was obtained as a white powder, and its molecular formula C15H26O, determined by HR-ESI-MS at m/z 245.1853 [M + Na]+ (calcd for C15H26ONa, 245.1881). The 1H-NMR spectrum of alpiblepharin A (1) displayed the characteristic resonances for four methyl singlets at 1.20 (3H, s, H-15), 1.16 (3H, s, H-14), and 1.61 (6H, s, H-11,12). The multiplet signal at 5.19-5.14 (2H, m) was assigned to the two methine protons H-2 and H-6. All this information from 13C NMR, as well as HMBC, HSQC led to the structure for this compound, which indicated the basic skeleton of 1 was similar to that of helminthogermacrene [19]. The chemical structure of 1 was elucidated as shown in Figure 1 and named triviallys alpiblepharin A.
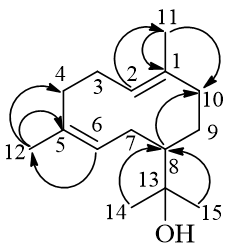
Fig. 1. Structure and the important HMBC of alpiblepharin A.
Table 1. Phytochemical composition of the seeds of A. blepharocalyx
|
No |
Compounds |
Bioactivities |
|
1 |
Alpiblepharin A |
Anti-inflammatory, anti-oxidative, anti-cancer effects |
|
2 |
Flavokawain A |
Anti-inflammatory, anti-oxidative, anti-cancer effects |
|
3 |
2', 6'-dihydroxy-4'-metoxychalcone |
Anti-oxidative, anti-cancer effects |
|
4 |
Nevadensin |
Anti-oxidative, anti-cancer effects |
|
5 |
Apigenin |
Anti-oxidative, anti-cancer, anticancer, anti-diabetic effects |
|
6 |
Apigetrin |
Anti-oxidative, anti-inflammatory, anticancer, anti-diabetic effects |
|
7 |
Cynaroside |
Anti-inflammatory, anti-oxidative effects |
|
8 |
Rutin |
Anti-inflammatory, anti-oxidative effects |
|
9 |
Polydatin |
Anti-inflammatory, anti-oxidative, anti-cancer effects |
Anti-inflammatory effect of compound 1
In this study, compound 1 displayed the most significant inhibition of superoxide anion generation and elastase release with IC50 values of 4.3 ± 0.3 and 6.3 ± 1.7μM, respectively.
Anti-cancer effect of compound 1-9
The purified compounds 1-9 were evaluated for in vitro cytotoxicity against KB, HepG2, SK-LU-1, and MCF7 cancer cell lines. The compounds 2-9 exhibited moderate activity that inhibited cancer cell lines with IC50 values of 37.00 ± 3.66 to 55.41 ± 2.16 μM/mL. Compound 1 showed significant cytotoxicity against cancer cell lines with IC50 values of 15.23 ± 2.14 to 25.81 ± 2.45 μM/mL. The cytotoxicity may be significant affected by the hydroxyl group. The esterification will decrease the cytotoxic activity of this compound. These data provided a scientific basis for further application of black shallot for the treatment of cancer-related diseases.
Conclusion
In this study, the presence of 9 compounds in A. blepharocalyx seed extract was identified, including alpiblepharin A, flavokawain A, 2', 6'-dihydroxy-4'-metoxychalcone, nevadensin, apigenin, apigetrin, cynaroside, rutin, polydatin. Moreover, the SAB extract exhibited a stronger anticancer and anti-inflammatory. Alpiblepharin A (1) was isolated from natural for the first time. This compound showed significant anti-inflammatory (IC50 values of 4.3 ± 0.3 and 6.3 ± 1.7μM) and cytotoxic activity against KB, SK-LU-1, MCF7, and HepG2 cancer cell lines (IC50 of 15.23 ± 2.14 to 25.81 ± 2.45 μM/mL). The data indicate Alpinia blepharocalyx as a potential food supplements for treating inflammatory and cancer-related diseases.
Acknowledgment
The authors gratefully acknowledge grants from the Ministry of Education and Training (MOET), Vietnam (No. B2018-TDV-09) for the financial support of the present research.
Conflict-of- interest Notification Page:
The authors declare there is no conflict of interest regarding the publication of this paper.
References
