CYTOTOXICITY OF GALANTAMINE PEPTIDE ESTERS AGAINST HELA CELL LINE
Dobrina Tsvetkova1*, Lyubomir Vezenkov2, Tchavdar Ivanov2, Dancho Danalev3, Ivanka Kostadinova4
|
|
|
ABSTRACT
In this study, the cytotoxic potential of newly synthesized peptide esters of Galantamine, namely GAL-VAL and GAL-LEU, was investigated against the human cervical adenocarcinoma cell line (HeLa) across a range of concentrations spanning from 1.875µM to 30µM. Notably, GAL-LEU demonstrated significant efficacy in suppressing HeLa cells, particularly at a concentration of 30 µM, where it elicited an impressive 86.81% reduction in cell viability. This effect was corroborated by a corresponding index of cell viability of 13.19%. Further analysis revealed that GAL-LEU possessed a substantially lower IC50 value of 23.63 µM compared to GAL-VAL, which exhibited an IC50 value of 31.95µM. These findings highlight the pronounced cytotoxic effects of both esters on the HeLa cell line, with GAL-LEU exhibiting superior antiproliferative potency. The results of this investigation suggest the potential utility of GAL-VAL and GAL-LEU as promising candidates warranting further exploration in cancer therapeutics, particularly in the context of addressing cervical adenocarcinoma.
Keywords: Peptide esters, Galantamine, MTT, HeLa cell line
Introduction
Galantamine is a specific long-acting centrally active competitive (reversible) inhibitor of the enzyme acetylcholinesterase [1, 2], having antioxidant properties and a positive allosteric modulatory action on nicotinic acetylcholine receptors of the α7-subtype [3-6].
It has been reported that L-Leucyl-L-Leucine methyl ester leads to apoptosis on in cell lines [7]. Similarly, in HCT-15 cells, N-phosphoryl dipeptide methyl esters may cause apoptosis [8] and (di-isopropyloxyphoryl-Trp)2-Lys-OCH3 inhibits the proliferation of HeLa cells [9].
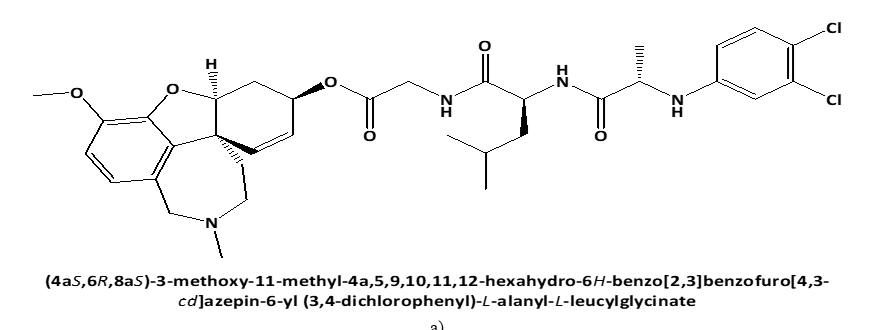
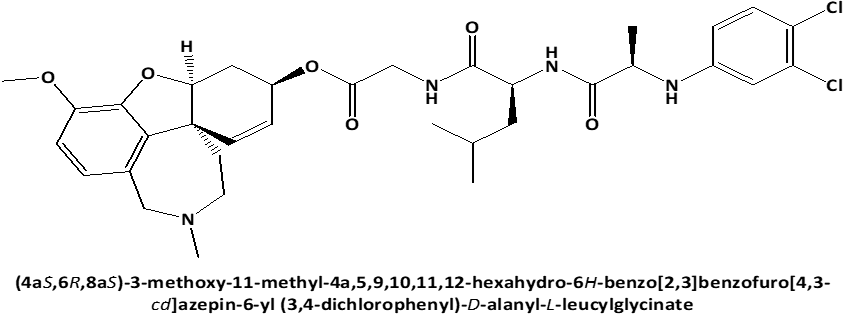
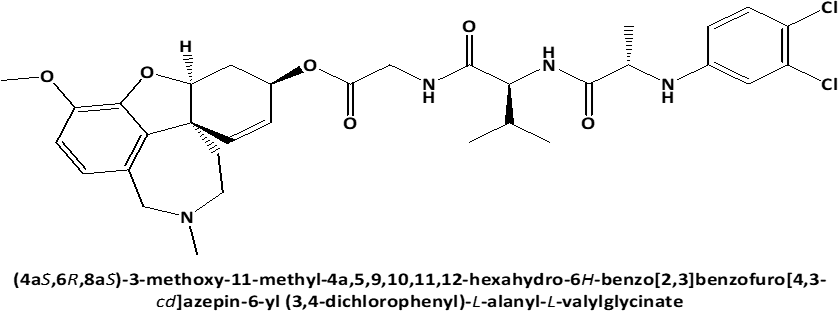
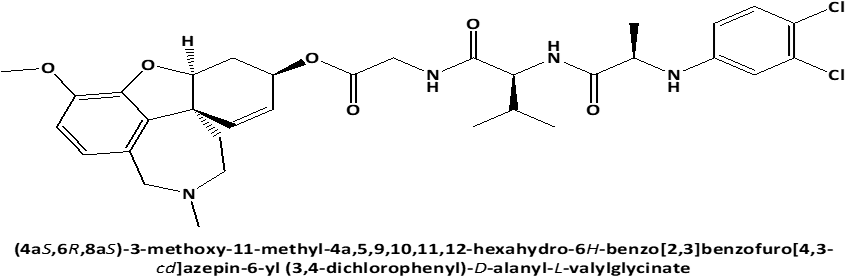
Following these data, the study aimed to investigate the cytotoxic effect on human cervical adenocarcinoma cell line (HeLa) of newly synthesized peptide esters of Galantamine: 6-O-N-[N-(3,4-chlorophenyl)-D, L-Alanyl]-L-Valil-Glycil-Galantamine (GAL-VAL) and 6-O-N-[N-(3,4-chlorophenyl)-D, L-Alanyl]-L-Leucyl-Glycil-Galantamine (GAL-LEU) [10], which, when used in the ferric reducing/antioxidant power (FRAP) technique, have both acetylcholinesterase and g-secretase inhibitory action [11, 12].
Materials and Methods
Materials
In experiments for the assessment of the cytotoxic activity of peptide esters against the HeLa cervical adenocarcinoma cell line, the medium Minimum Essential Medium Eagle was used.
 |
|
a) |
 |
|
b) |
 |
|
c) |
 |
|
d) |
|
Figure 1. Chemical structures of GAL-LEU and GAL-VAL. |
Fetal bovine serum (FBS), 100 IU/ml of penicillin, 100 µg/ml of Streptomycin, standard MTT (3-[4.5-dimethylthiazole-2-yl]-2.5-diphenyl-tetrazolium bromide), dimethylsulfoxide, 0.25% Trypsin EDTA 1X.
The examined peptide esters of Galantamine GAL-LEU and GAL-VAL were dissolved separately in dimethylsulfoxide to obtain stock solutions of each ester with a concentration of 20 mM.
The МТТ solution was primed by dissolving MTT in phosphate buffer solution to obtain a solution with a concentration of MTT 5 mg/ml. This solution is stable for 1 month at storage at 4 °С.
Methods
In 96-well microplates, the cytotoxic activity of several substances was assessed. 100 µg/ml Streptomycin, 100 IU/ml Penicillin, and 5% of fetal bovine serum were added to Minimum Essential Medium Eagle, which was used to cultivate HeLa cervical cancer cells in 75 cm2 flasks at 37 °C in a 5% CO2 thoroughly humidified atmosphere. Exponentially growing cells were trypsinized, centrifuged, and a hemocytometer was utilized to count them. To get the concentration of: 6x104 HeLa cells/ml, the cell suspension was diluted with culture media. 100 µl of cell suspension were introduced into all wells and were incubated for 24 h at 37°C in a fully humidified atmosphere of 5% CO2. The culture medium was removed after incubation.
MTT-test of Mosmann was applied [13]. 200 µl of solution of respective ester in media were added separately in different intensities (1.875 µM – 30 µM) into each well. Experiments were performed in triplicates for each ester in each concentration and the average values were calculated. 200 µl of MTT (0.5 mg/ml) was added to each well after 48 hours. For the incubation of the plates for 4 h at 37oC, a 5% CO2 incubator was used. After removing of supernatant from each well were added 100 µl of dimethylsulfoxide to solubilize the formed formazan, with an absorbance of λ = 570 nm.
The cytotoxicity was measured as the concentration that prevented HeLa cells from growing by 50% (IC50). Index cell viability V (%) and cell growth inhibition I (%) by the following equations were calculated:
|
|
(1) |
|
|
(2) |
V (%) – index cell viability
I (%) – cell growth inhibition
At – mean absorbance value of formazan, obtained after treatment of HeLa cells with test compound
A(+) – mean absorbance of formazan, obtained with the positive control (HeLa cell line MTT-treated without the investigated substances )
A(-) – mean absorbance value of formazan, obtained with negative control (MTT, without the addition of the examined compounds).
Results and Discussion
In order to determine IC50 of GAL-LEU and GAL-VAL against the HeLa cell line the extrapolation of the data for the absorbances of formazan and concentration of added ester is made. As a standard was used Doxorubicin. Absorbances of positive: A (+) and negative: A (–) controls and of formazan obtained after treatment of HeLa cells with tested compounds are reported in Таble 1.
Таble 1. Absorbances controls and of formazan produced from the GAL-LEU- and GAL-VAL-treated HeLa cell line.
|
N: |
A (+) |
A (–) |
|||
|
1 |
1.649 |
0.085 |
|||
|
2 |
1.571 |
0.084 |
|||
|
3 |
1.672 |
0.081 |
|||
|
4 |
1.630 |
0.084 |
|||
|
5 |
1.792 |
|
|||
|
6 |
1.827 |
|
|||
|
X |
1.690 |
0.084 |
|||
|
SD |
0.099 |
0.002 |
|||
|
Formazan Absorbance [AU] |
|||||
|
CGAL- LEU [mM] |
1. |
2. |
3. |
X |
SD |
|
1.875 |
1.421 |
1.553 |
1.570 |
1.515 |
0.081 |
|
3.75 |
1.398 |
1.455 |
1.488 |
1.447 |
0.046 |
|
7.5 |
1.453 |
1.503 |
1.498 |
1.485 |
0.028 |
|
15 |
1.332 |
1.212 |
1.352 |
1.299 |
0.076 |
|
30 |
0.200 |
0.466 |
0.220 |
0.295 |
0.148 |
|
CGAL-VAL [mM] |
Formazan Absorbance [AU] |
||||
|
1 |
2 |
3 |
X |
SD |
|
|
1.875 |
1.573 |
1.684 |
1.688 |
1.648 |
0.065 |
|
3.75 |
1.597 |
1.684 |
1.611 |
1.631 |
0.047 |
|
7.5 |
1.483 |
1.536 |
1.602 |
1.540 |
0.060 |
|
15 |
1.438 |
1.516 |
1.512 |
1.489 |
0.044 |
|
30 |
1.125 |
1.241 |
1.23 |
1.199 |
0.064 |
Figure 2 illustrates the correlation between the concentration of peptide esters and the formazan absorbance.
|
|
|
|
a) |
b) |
|
Figure 2. Relation between the absorbance of formazan and concentration of GAL-LEU and GAL-VAL. |
|
Effect of different concentrations (1.875 mМ – 30 mМ) GAL-LEU and GAL-VAL on the survival of HeLa cells is shown in Таble 2.
Таble 2. Index of HeLa cell viability after treatment with GAL-LEU and GAL-VAL.
|
CGAL- LEU [mM] |
Index of HeLa cell viability [%] |
||||
|
1 |
2 |
3 |
X |
SD |
|
|
1.875 |
83.25 |
91.46 |
92.52 |
89.08 |
5.07 |
|
3.75 |
81.82 |
85.36 |
87.42 |
84.87 |
2.83 |
|
7.5 |
85.24 |
88.35 |
88.04 |
87.21 |
1.71 |
|
15 |
77.71 |
70.24 |
78.95 |
75.63 |
4.71 |
|
30 |
7.25 |
23.81 |
8.5 |
13.19 |
9.22 |
|
CGAL-VAL [mM] |
Index of HeLa cell viability [%] |
||||
|
1 |
2 |
3 |
X |
SD |
|
|
1.875 |
92.71 |
99.62 |
99.87 |
97.4 |
4.06 |
|
3.75 |
94.2 |
99.62 |
95.07 |
96.3 |
2.91 |
|
7.5 |
87.11 |
90.4 |
94.51 |
90.67 |
3.71 |
|
15 |
84.3 |
89.16 |
88.91 |
87.46 |
2.74 |
|
30 |
64.82 |
72.04 |
71.36 |
69.41 |
3.99 |
The accordance between the concentration of peptide esters and the index of HeLa cell viability is demonstrated in Figure 3.
|
|
|
|
a) |
b) |
|
Figure 3. HeLa cells survival after treatment with GAL-LEU and GAL-VAL. |
|
Experimental results from the investigation of the cytotoxic activity of peptide esters against HeLa cells are presented in Таble 3.
Таble 3. Effect of GAL-LEU and GAL-VAL on HeLa cell line proliferation.
|
CGAL-LEU [mM] |
Inhibition of Hela cell growth [%] treated with GAL-LEU |
||||
|
1 |
2 |
3 |
X |
SD |
|
|
1.875 |
16.75 |
8.54 |
7.48 |
10.92 |
5.07 |
|
3.75 |
18.18 |
14.64 |
12.58 |
15.13 |
2.83 |
|
7.5 |
14.76 |
11.65 |
11.96 |
12.79 |
1.71 |
|
15 |
22.29 |
29.76 |
21.05 |
24.37 |
4.71 |
|
30 |
92.75 |
76.19 |
91.50 |
86.81 |
9.22 |
|
IC50 |
23.29 |
23.98 |
23.62 |
23.63 |
0.35 |
|
CGAL-VAL [mM] |
Inhibition of Hela cell growth [%] treated with GAL-VAL |
||||
|
1 |
2 |
3 |
X |
SD |
|
|
1.875 |
7.29 |
0.38 |
0.13 |
2.60 |
4.06 |
|
3.75 |
5.80 |
0.38 |
4.93 |
3.70 |
2.91 |
|
7.5 |
12.89 |
9.60 |
5.49 |
9.33 |
3.71 |
|
15 |
15.70 |
10.84 |
11.09 |
12.54 |
2.74 |
|
30 |
35.18 |
27.96 |
28.64 |
30.59 |
3.99 |
|
IC50 |
35.20 |
30.19 |
30.46 |
31.95 |
2.82 |
The suppression of HeLa cells by peptide esters is presented in Figure 4.
|
|
|
|
a) |
b) |
|
Figure 4. Cytotoxic effect of GAL-LEU and GAL-VAL against Hela cell lines. |
|
The obtained data for inhibition concentration 50 % (IC50) are GAL-LEU (23.63 mM, GAL-VAL (31.95 mM), and standard Doxorubicin (3.1 mM).
Due to its benefits, including being quick, adaptable, quantitative, highly repeatable, and sensitive, the MTT test of Mosmann has been widely used to investigate the cytotoxic activities of various substances. The MTT assay measures a viable cell's capacity to change a soluble yellow tetrazolium salt [3-(4.5-dimethylthiazol-2-yl)-2.5-diphenyltetrazolium bromide] (MTT) into an insoluble violet formazan crystal that can be dissolved in an organic solvent and can be detected spectrophotometrically at λ = 570 nm. Tetrazolium salt accepts electrons from oxidized substrates or appropriate enzymes. Due to succinate dehydrogenase activity, MTT is decreased at the ubiquinone, cytochrome B, and cytochrome C sites of the mitochondrial electron transport system. The conversion may be directly tied to the amount of live cells since the reduction only occurs when mitochondrial reductase enzymes are functioning. The quantity and absorbance of formazan increase as the number of cells increases. A dose-response curve is created when the quantity of formazan generated by cells treated with an agent is compared to the amount produced by control cells left untreated. This comparison allows one to determine how well the agent kills cells [13].
MTT assay is applied for the determination of cytotoxicity of different extracts, such as of Acalypha wilkesiana against breast cancer cell MCF-7 [14], and human cervical cancer cell line HeLa [15] and of Rheum ribes L. towards KB and A-549 cell lines [16].
Cancers are malignant tumors that invade tissues [17]. It is known that after mutation stem cells can form cancer [18]. Cervical cancer, Sickle-cell anaemia and thalassemia are spread in Saudi Arabia [19].
The epithelial carcinoma HeLa is the first type of human cancer cell line, which has been cultured continuously. HeLa was isolated from the cervical cancer of woman in 1951. Till now the knowledge of many processes occurring in organism cells has been observed applying HeLa line [20].
In cell culture process cells from different cell line grow in a specific mediun [21]. Dsouza in current study HeLa cells are cultivated in Minimum Essential Medium Eagle. As a standard was used Doxorubicin that is known to inhibit phosphatidylserine [22] decarboxylase and changes mitochondrial membrane in HeLa cells [23].
HeLa cell line was treated separately with each of the examined peptide esters in different concentrations (1.875 mМ – 30 mМ) and MTT assay was evaluated. Experimental data show that the treatment of HeLa cells resulted in a reduced concentration of formazan, which indicates a growth-inhibitory property of esters.
From the experimental results it was observed that the cytotoxicity of GAL-LEU at concentration 30 mM is 86.81 % and the index of cell viability is 13.19 %. In comparison, the lower antiproliferative effect of GAL-VAL is proved by the fact that in concentration 30 mM with a 69.41% index of cell survival, it inhibits 30.59% of cell proliferation. The cytotoxicity is measured as the concentration that inhibits cell growth by 50% (IC50). It was reported that 0.1 μM Doxorubicin reduced the survival of Hela cells to 40 % [24].
Experimental results for cell growth inhibition are plotted on non-linear regression analysis using Microsoft Exel Programm and data for IC50 are calculated from the respectively obtained equation: GAL-LEU (IC50 = 23.63 μM ± 0.35 μM) (y = 9.278.e0.071.x) and GAL-VAL (IC50 = 31.95 μM ± 2.82 μM) (y = 3.140.e0.081.x). Results for IC50 for GAL-LEU (IC50 = 23.63 μM) and GAL-VAL (IC50 = 31.95 μM) are higher than the experimentally obtained data for standard Doxorubicin (IC50 = 3.1 μM). These results proved that the examined peptide esters possess lower antiproliferative activity than standard Doxorubicin.
It has been reported that Ledakrin produces a dose-dependent suppression of DNA synthesis and replication in HeLa S3 cells by inhibition of thymidine incorporation [25]. It has been described that the inhibitor of phosphatidylinositol 3-kinases promotes mitotic cell death in HeLa cells [26]. Anticancer activity on HeLa cell lines of coumarin derivative Calanone, isolated from genus Calophyllum (IC50 = 22.887 μg/ml) [27] and of ethanolic extract of Canthium Parviflorum Lam [IC50 = 43.15 μg/ml] [28] has been observed.
It has been demonstrated that the inhibitory effect on the HeLa cell line of an antitumor triterpenoid: cyano enone of methyl boswellates at 1 μM is 78.5 % and IC 50 = 0.27 μM [29].
It has been shown that DNA methyltransferase inhibitor Zebularine [30] and synthetic antioxidants butylated hydroxyanisole [31] and propylgallate [32] inhibit the growth of HeLa cells via caspase-dependent apoptosis, as a result of activation caspase 3 and caspase 8. The observed data for IC50 are respectively: Zebularine (IC50 = 130 μM) [30], butylated hydroxyanisole (IC50 = 150 μM) [31], propylgallate (IC50 = 800 μM) [32]. The low value of IC50 corresponds to high cytotoxic activity. From the presented results it is obvious that triterpenoid is more effective in the suppression of cell proliferation, followed by Zebularine, butylated hydroxyanisole, and propylgallate.
(DIPP-Trp)2-Lys-OCH3 produced a dose-dependent inhibition of HeLa cell growth. 100 μM (DIPP-Trp)2-Lys-OCH3 inhibits 85% of HeLa cells. The inhibitory concentration of 50 % (IC50) is 42.23 μM [9].
In comparison with these data from our experimental results is obvious that: 1) GAL-LEU possesses activity similar to this of coumarin derivative Calanone; 2) GAL-LEU (IC50 = 23.63 μM) and GAL-VAL (IC50 = 31.95 μM) are more active on HeLa cells in comparison with Zebularine, butylated hydroxyanisole and propylgallate.
Conclusion
Reduced formazan concentration and absorbance after the esters treatment show that they have growth-inhibiting properties. The experimental results for inhibitory concentration IC50 are: 23.63 mM (GAL-LEU) and 31.95 mM (GAL-VAL) demonstrating that both esters have cytotoxic effects on the HeLa cell line and that, compared to GAL-VAL, GAL-LEU has a stronger antiproliferative impact because of its lower IC50 value.
Acknowledgments: The authors are gratefully acknowledged Professor Iqbal Choudhary Sadia Siddiq and Rizwana Malik from ”Dr. Panjwani Center for Molecular Medicine and Drug Research, ICCBS, University of Karachi, Pakistan, for skillful technical assistance, great experimental support, extending laboratory competence, and for their continued scientific correspondence, cooperation, and help. The authors gratefully acknowledged Prof. Danka Obreshkova for scientific consultation and cooperation.
Conflict of interest: None
Financial support: None
Ethics statement: None
