DESIGN, SYNTHESIS, COMPUTATIONAL STUDY, AND BIOLOGICAL EVALUATION OF 6-ARYL SUBSTITUTED PYRIMIDINE SCHIFF BASES
Aziz Unnisa1*, Abouzied Amr S2a, b, Anupama B3, Chenchu Lakshmi K N V4, Ramadevi Kunduru5, Kesavanarayanan K S6
|
|
|
ABSTRACT
Context: Schiff bases (SB) is class of molecules that possess a wide spectrum of biological activities.Aims: Thus, the present study focuses on the synthesis and characterization of novel Schiff bases, which have not previously been explored in the literature and to investigate the synthesized compounds for anti-inflammatory and antioxidant activities with no or fewer side effects. Settings and Design: A group of 6-aryl substituted pyrimidine Schiff bases were synthesized by condensation of 6-phenyl pyrimidine 2-amine with different aldehydes. The synthetic compounds were studied for their in-vitro anti-inflammatory and antioxidant activities. Methods and Material: The characterization of the synthesized candidates was performed using 1H NMR, 13C NMR, IR, and MS. Computational study of designed compounds was done by OCHEM, Molinspiration cheminformatics, Datawarrior, and Swiss ADMET. DPPH assay was used to determine antioxidant activity and heat hemolysis method for anti-inflammatory activity.Results: All the test compounds showed dose-dependent inhibition, compound 5a showed consistent antioxidant and anti-inflammatory activities. Computational studies predicted that none of the compounds can cross BBB, non-mutagenic, showed moderate activity against ion channel modulator, GPCR ligand, nuclear receptor, and enzymes. Conclusions: All the synthesized compounds, pyrimidine SB exhibited antioxidant and anti-inflammatory activities with less toxicity. Amongst the titled compounds, 5a and 5f were found to greatly influence the activity which may be due to the type of substituents present on the ring. The study carried out considered the three-dimensional nature of chemical structures that played an important part in ligand-receptor binding and assisted in providing an approach for further optimization of new leads.
Keywords: Pyrimidine Schiff bases, DPPH assay, heat hemolysis, BOILED egg.
Introduction
Schiff bases (SB) are organic compounds formed by condensation of an amine with aldehyde or ketone. The imine fragment is essential for the biological actions of Schiff bases.[1] Several SB has been studied for their significant biological activities like antioxidant, anti-inflammatory agents, antitumor, antiviral, insecticidal, antibacterial, antitubercular, antimicrobial, anticonvulsant activity, antimalarial, antiproliferative, and antipyretic activities, etc. Health is an important factor in human beings’ life. [2-5]
In the present scenario, several investigations are in progress on free radicals which are responsible for the formation of inflammatory mediators that trigger a huge variety of human diseases such as atherosclerosis, neurodegenerative diseases, cancer initiation, age-related diseases, tumors, [6-8] and rheumatoid arthritis due to their interaction with biological systems. Antioxidants generally act as H- donors or electron donors to the reactive site for neutralizing free radicals. [9, 10] Thus, it seems worthwhile to continue to investigate compounds with anti-inflammatory and antioxidant activities with no or fewer side effects.
The literature revealed that SB is a class of molecules that possess both these activities. Therefore, the present study focuses on the synthesis and characterization of novel Schiff bases, which have not previously been explored in the literature.
Many chemists have investigated the synthesis of pyrimidine derivatives by different methods. An imine containing pyrimidine derivatives have been synthesized with modified procedures. [11-13] Pyrimidine derivatives attract great interest due to the wide variety of biological actions possed by these compounds, such as anticancer, [14, 15] antitumor, [16] antidiabetic, [17] anti-inflammatory, [18-20] antimicrobial, [21-25] antifungal, [26] and antioxidant activities. [27, 28]
As described above, we decided to synthesize novel SB containing a pyrimidine ring in three steps employing frictional force and conventional method in the preparation of titled compounds depicted in Fig 1. Insights into the drug-like characters of these compounds were provided using computational methods such as OCHEM, Molinspiration, OSIRIS Property Explorer, Swiss-ADMET.
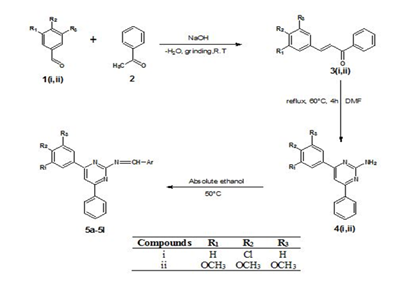
Figure 1: Synthesis of Schiff bases
Materials and Methods
The laboratory reagent grade chemicals were used without further purification. Melting points were determined in open glass capillaries using Tempo (600) melting point apparatus. IR spectra obtained from Bruker analyzers were established by Shimadzu FT-IR Spectrophotometer using the KBr pellets, Model No.8400S (Japan). 1H and 13C NMR spectra were documented on Bruker 400 MHz NMR spectrometer (Switzerland) using DMSO as a solvent. TLC was run on silica gel G plates using n-hexane: ethyl acetate (5:5) as a mobile phase to assess the reaction progression and purity of synthesized compounds. Shimadzu 1800 double beam UV-Visible spectrophotometer with a wavelength accuracy of 0.5 nm and a pair of 1 cm matched quartz cell was used for the measurement of optical density values. Centrifuge (REMI-C854/8) was used for centrifugation.
Synthesis of substituted chalcones
Equimolar of acetophenone (0.01 mol) and different aromatic benzaldehyde (0.01 mol) was taken into a mortar, NaOH was added and ground by pestle at room temperature by employing the friction method. Then the mixture was moistened with water. The reaction progression was checked by TLC, and all the reactions were observed to be completed in a time range of 15–20 min. The product was recrystallized from methanol and the purity was confirmed by melting point and TLC.
General Procedure for Synthesis of substituted Phenyl pyrimidine-2-amine derivatives
Equimoles of substituted Chalcones (0.01 mol) and Guanidine HCl (0.01 mmol) were refluxed for 4hrs in the presence of DMF by maintaining the temperature between 50-600C in a water bath. The mixture was moved into ice-cold water, the precipitate was filtered, dried, and recrystallized from methanol. The purity and progress of the reaction were confirmed by TLC and melting point.
General Procedure for Synthesis of SB
Compounds 5a-5l (Table 1) were prepared by a conventional method. Equimolar quantities of Substituted phenyl pyrimidine-2-amine derivatives were condensed with the corresponding aldehyde in absolute ethanol (20 mL) in the presence of sodium hydroxide. The reaction mixture was stirred on a magnetic stirrer for 15-20 mins and the contents of the flask were poured over crushed ice. Evaporation of the excess solvent afforded the desired product in the 75-85% yield. The product was filtered, washed, dried, and recrystallized with methanol.
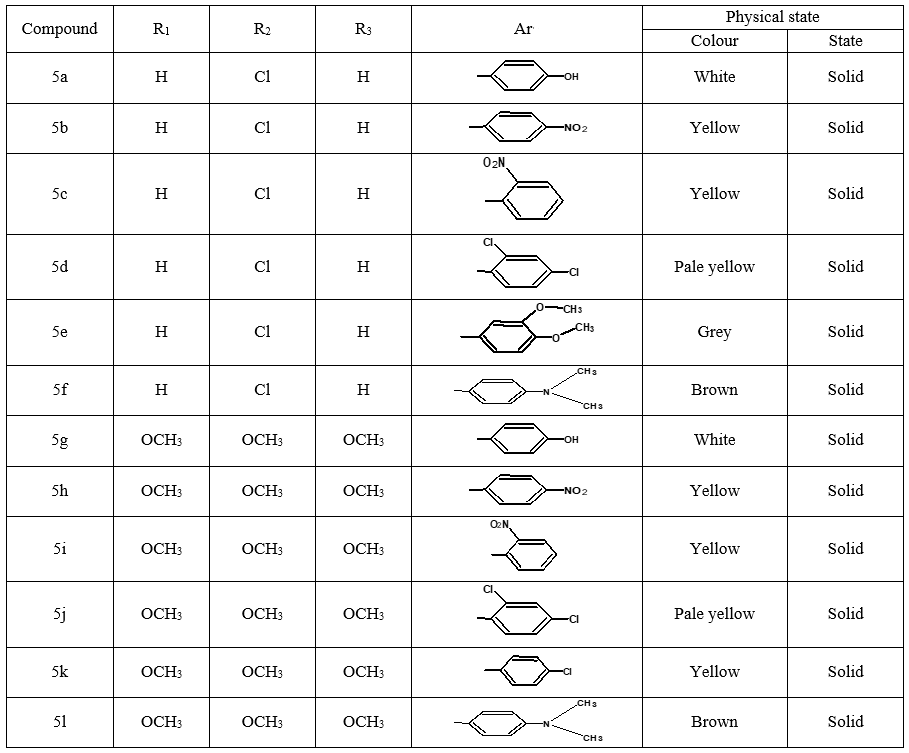
Table 1: Physical data of Schiff bases
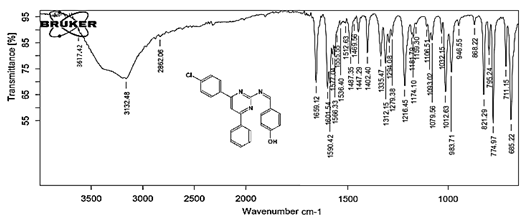
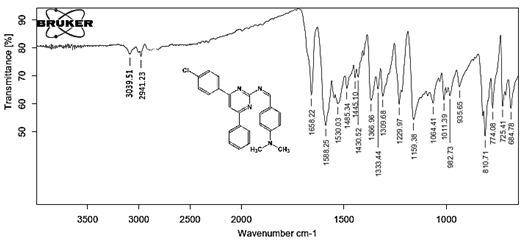
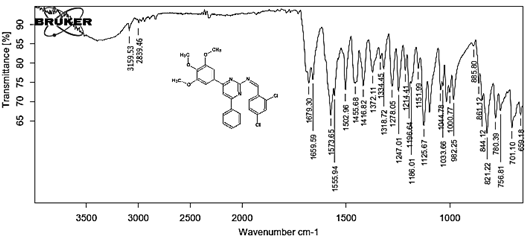
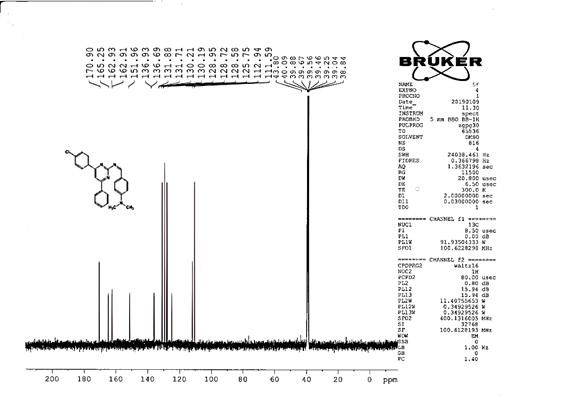
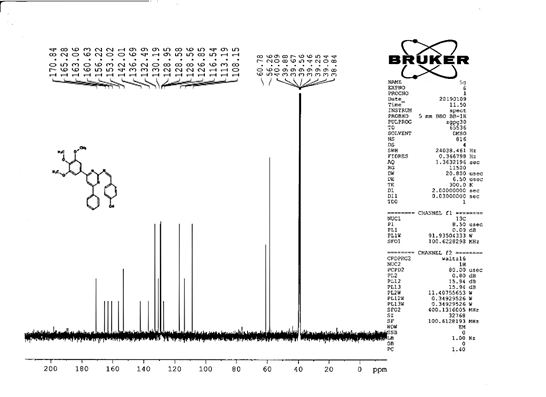
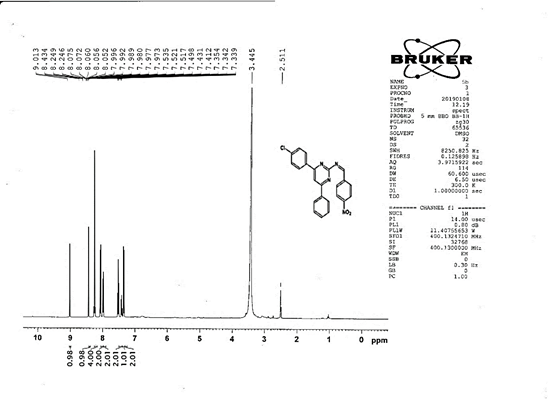
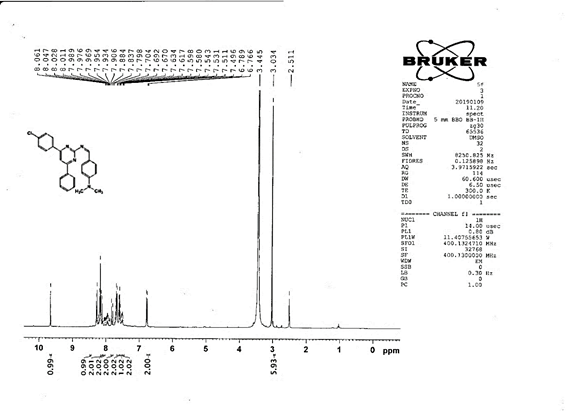
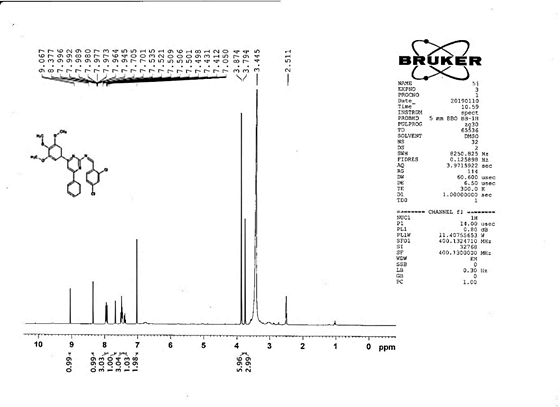
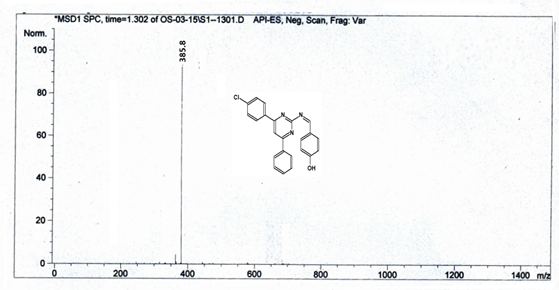
4-[(Z)-{[4-(4-chlorophenyl)-6-phenylpyrimidin-2-yl]imino}methyl]phenol 5a. Molecular formula: C23H16ClN3O; Composition: C(71.59%), H(4.18%), Cl(9.19%), N(10.89%), O(4.15%); Yield: 76.32%; M.P: 208℃ ; 1H NMR (400 MHz, DMSO-d6) δ 9.01 (s, 1H), 8.43 (s, 1H), 8.13 (s, 1H), 8.10 – 8.03 (m, 2H), 8.02 – 7.95 (m, 2H), 7.83 – 7.76 (m, 2H), 7.56 – 7.47 (m, 2H), 7.41 (ddt, J = 8.0, 6.7, 1.5 Hz, 1H), 7.38 – 7.31 (m, 2H), 6.98 – 6.91 (m, 2H);13C NMR (100 MHz, DMSO-d6) δ 221.85 – 133.79 (m), 132.88, 131.43, 130.47, 130.09, 129.49, 129.18, 128.69 (d, J = 16.5 Hz), 115.41, 111.29; FT-IR (KBr, v max (cm-1)): 3132 (Ar–CH), 2862 (Alif.–CH), 1279 (C–N), 1659 (C=N), 3617 (O–H), 1294 (C–Cl); ESI-MS: 385.8 [M+H]+.
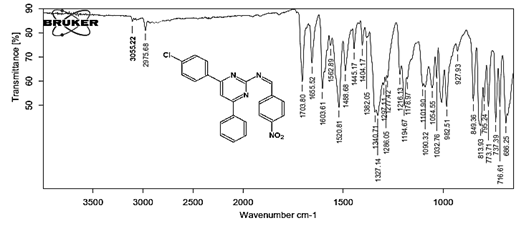
(Z)-N-[4-(4-chlorophenyl)-6-phenylpyrimidin-2-yl]-1-(4-nitrophenyl)methanimine 5b. Molecular formula: C23H15ClN4O2; Composition: C(66.59%), H(3.64%), Cl(8.55%), N(13.51%), O(7.71%); Yield: 80.15 %; M.P: 180℃; 1H NMR (400 MHz, DMSO-d6) δ 9.01 (s, 1H), 8.43 (s, 1H), 8.25 (d, J = 1.2 Hz, 4H), 8.10 – 8.03 (m, 2H), 8.02 – 7.95 (m, 2H), 7.56 – 7.47 (m, 2H), 7.41 (ddt, J = 8.0, 6.7, 1.5 Hz, 1H), 7.38 – 7.31 (m, 2H); 13C NMR (100 MHz, DMSO-d6) δ 170.55, 165.25, 162.92 (d, J = 2.5 Hz), 149.30, 138.99, 136.93, 136.69, 131.88, 130.82, 130.20 (d, J = 1.5 Hz), 128.95, 128.65 (d, J = 13.7 Hz), 124.60, 112.94; FT-IR (KBr, v max (cm-1)): 3055 (Ar–CH), 2975 (Alif.–CH), 1277 (C–N), 1655 (C=N), 1488 & 1340 (N=O), 1297 (C–Cl); ESI-MS: 414.8 [M+H]+.
(Z)-N-[4-(4-chlorophenyl)-6-phenylpyrimidin-2-yl]-1-(2-nitrophenyl)methanimine 5c. Molecular Formula: C23H15ClN4O2; Composition: C(66.59%), H(3.64%), Cl(8.55%), N(13.51%), O(7.71%); Yield: 84.62%; M.P: 178℃; 1H NMR (400 MHz, DMSO-d6) δ 9.18 (s, 1H), 8.43 (s, 1H), 8.10 – 8.01 (m, 4H), 8.01 – 7.95 (m, 2H), 7.73 (td, J = 7.4, 1.5 Hz, 1H), 7.65 (td, J = 7.5, 1.6 Hz, 1H), 7.56 – 7.47 (m, 2H), 7.41 (ddt, J = 8.0, 6.7, 1.5 Hz, 1H), 7.38 – 7.31 (m, 2H); 13C NMR (100 MHz, DMSO-d6) δ 165.14, 164.37, 162.92 (d, J = 2.5 Hz), 149.70, 136.93, 136.69, 131.95 (d, J = 13.5 Hz), 131.25, 130.79, 130.20 (d, J = 1.5 Hz), 129.05 – 128.48 (m), 124.61, 112.94; FT-IR (KBr, v max (cm-1)): 3034 (Ar–CH), 2935 (Alif.–CH), 1271 (C–N), 1657 (C=N), 1486 & 1342 (N=O), 1292 (C–Cl); ESI-MS: 414.8 [M+H]+.
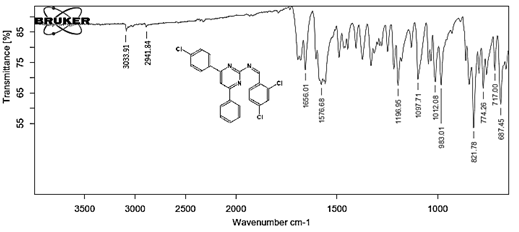
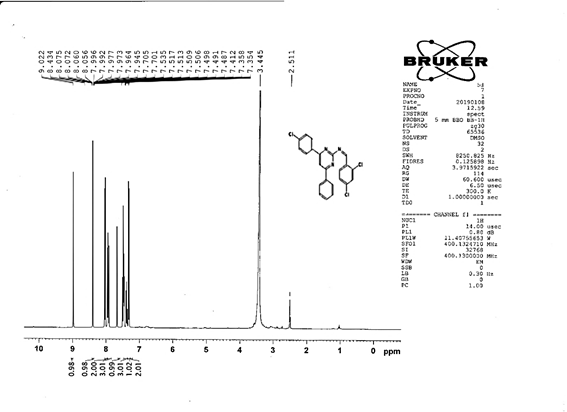
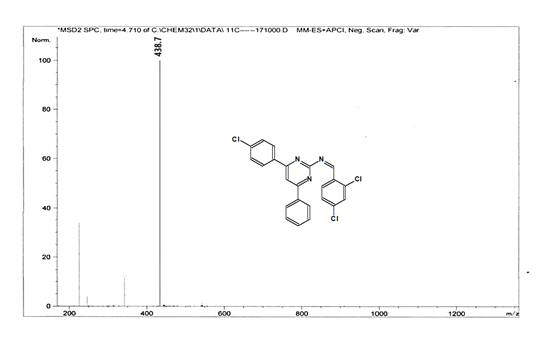
(Z)-N-[4-(4-chlorophenyl)-6-phenylpyrimidin-2-yl]-1-(2,4-dichlorophenyl) methanimine 5d. Molecular Formula: C23H14Cl3N3; Composition: C(62.96%), H(3.22%), Cl(24.24%), N(9.58%); Yield: 79.03%; M.P: 162℃; 1H NMR (400 MHz, DMSO-d6) δ 9.02 (s, 1H), 8.43 (s, 1H), 8.10 – 8.03 (m, 2H), 8.02 – 7.92 (m, 3H), 7.70 (d, J = 1.5 Hz, 1H), 7.56 – 7.46 (m, 3H), 7.41 (ddt, J = 7.9, 6.7, 1.5 Hz, 1H), 7.38 – 7.31 (m, 2H); 13C NMR (100 MHz, DMSO-d6) δ 165.73, 164.38, 162.92 (d, J = 2.5 Hz), 138.44, 136.93, 136.69, 135.70, 131.88, 131.25, 131.03, 130.20 (d, J = 1.5 Hz), 129.44, 128.95, 128.82 – 128.48 (m), 112.94; FT-IR (KBr, v max (cm-1)): 3033 (Ar–CH), 2941 (Alif.–CH), 1278 (C–N), 1656 (C=N), 1296 (C–Cl); ESI-MS: 438.7 [M+H]+.
(Z)-N-[4-(4-chlorophenyl)-6-phenylpyrimidin-2-yl]-1-(3,4-dimethoxyphenyl) methanimine 5e. Molecular Formula: C25H20ClN3O2; Composition: C(69.85%), H(4.69%), Cl(8.25%), N(9.77%), O(7.44%); Yield: 82.09%; M.P: 136℃; 1H NMR (400 MHz, DMSO-d6) δ 9.00 (s, 1H), 8.43 (s, 1H), 8.10 – 8.03 (m, 2H), 8.02 – 7.95 (m, 2H), 7.62 (dd, J = 7.4, 1.4 Hz, 1H), 7.56 – 7.47 (m, 3H), 7.41 (ddt, J = 7.9, 6.7, 1.5 Hz, 1H), 7.38 – 7.31 (m, 2H), 7.15 (d, J = 7.5 Hz, 1H), 3.83 (d, J = 0.9 Hz, 6H); 13C NMR (100 MHz, DMSO-d6) δ 169.33, 165.27, 162.92 (d, J = 2.5 Hz), 152.39, 150.56, 136.93, 136.69, 131.88, 130.20 (d, J = 1.4 Hz), 129.03 (d, J = 16.3 Hz), 128.65 (d, J = 13.7 Hz), 125.81, 112.94, 112.42 (d, J = 14.6 Hz), 55.89 (d, J = 2.8 Hz); FT-IR (KBr, v max (cm-1)): 3036 (Ar–CH), 2942 (Alif.–CH), 1266 (C–N), 1656 (C=N), 1032 & 1242 (C–O), 1295 (C–Cl); ESI-MS: 429.8 [M+H]+.
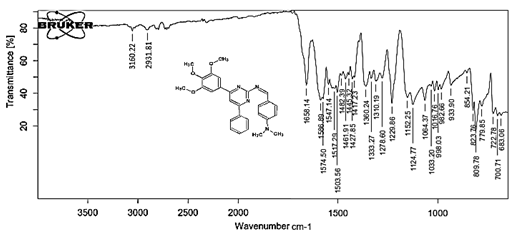
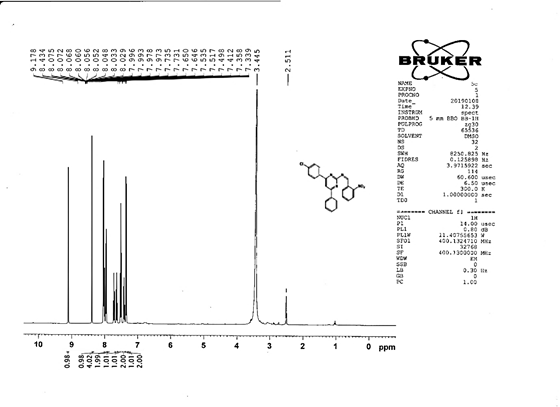
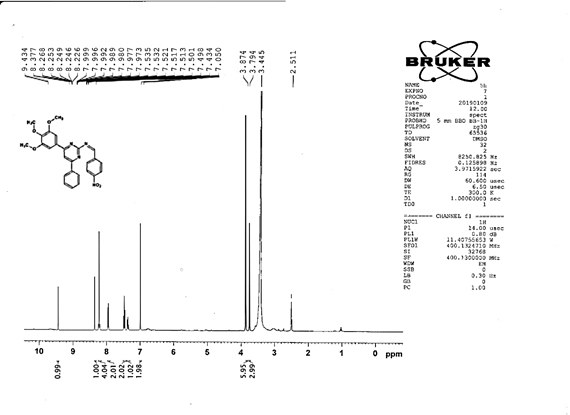
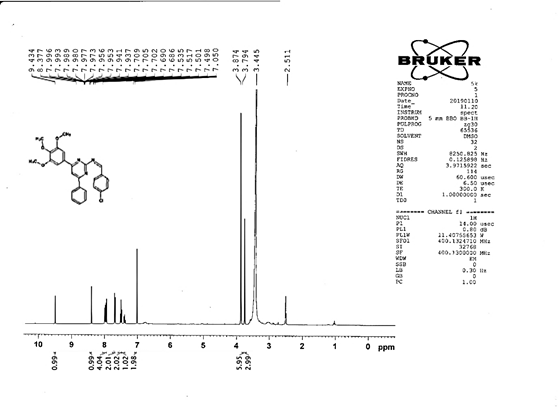
4-[(Z)-{[4-(4-chlorophenyl)-6-phenylpyrimidin-2-yl]imino}methyl]-N,Ndimethyl aniline 5f. Molecular Formula: C22H21ClN4; Composition: C(72.72%), H(5.13%), Cl(8.59%), N(13.57%); Yield: 76.39%; M.P: 164℃; 1H NMR (400 MHz, DMSO-d6) δ 9.01 (s, 1H), 8.43 (s, 1H), 8.10 – 8.03 (m, 2H), 8.02 – 7.95 (m, 2H), 7.76 – 7.68 (m, 2H), 7.56 – 7.47 (m, 2H), 7.41 (ddt, J = 7.9, 6.7, 1.5 Hz, 1H), 7.38 – 7.32 (m, 2H), 6.74 – 6.67 (m, 2H), 3.00 (s, 6H); 13C NMR (100 MHz, DMSO-d6) δ 170.90, 165.25, 162.92 (d, J = 2.5 Hz), 151.96, 136.93, 136.69, 131.80 (d, J = 17.1 Hz), 130.20 (d, J = 1.5 Hz), 128.95, 128.65 (d, J = 13.7 Hz), 125.75, 112.94, 111.59, 40.27; FT-IR (KBr, v max (cm-1)): 3039 (Ar–CH), 2941 (Alif.–CH), 1229 (C–N), 1658 (C=N), 1309 (C–Cl); ESI-MS: 412.9 [M+H]+.
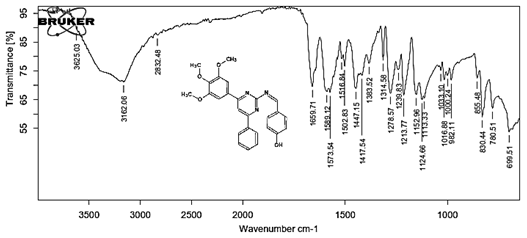
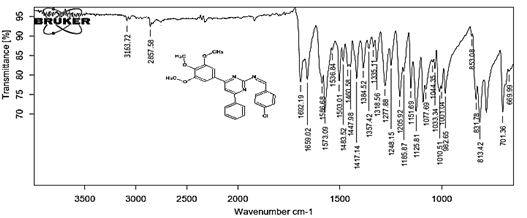
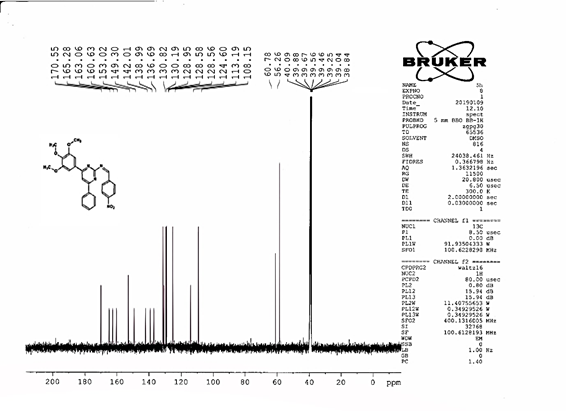
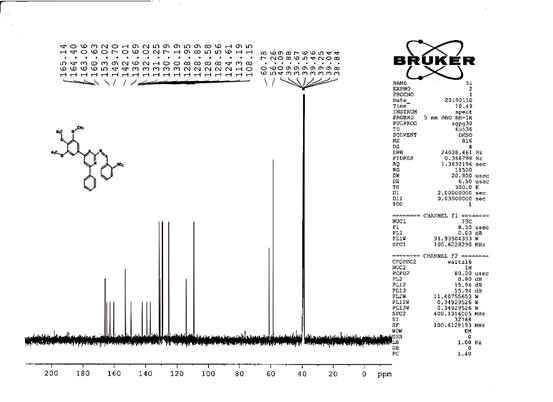
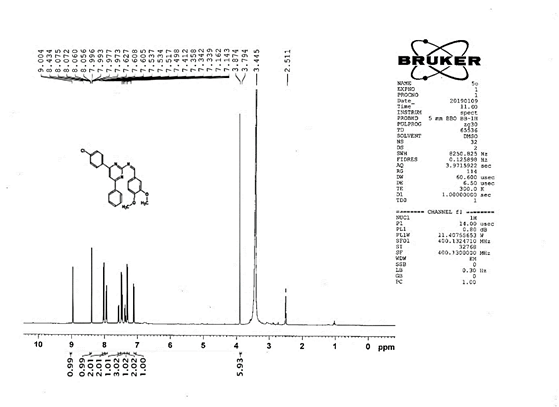
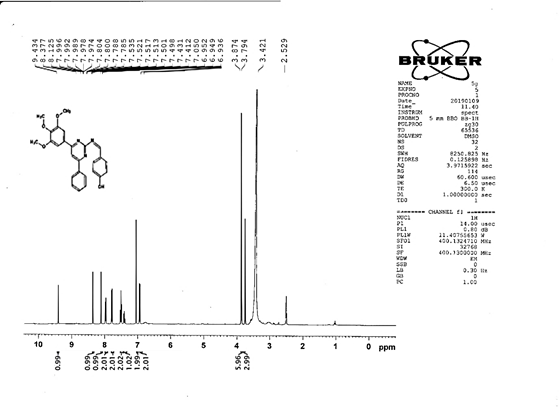
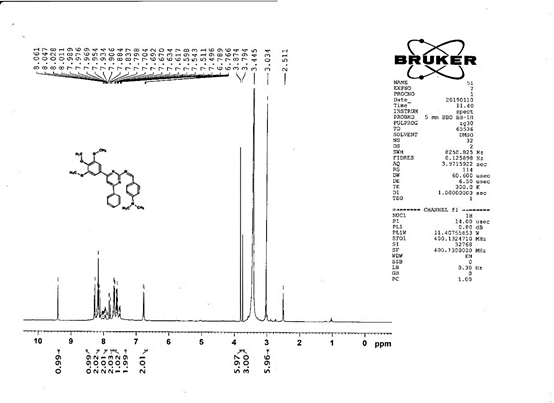
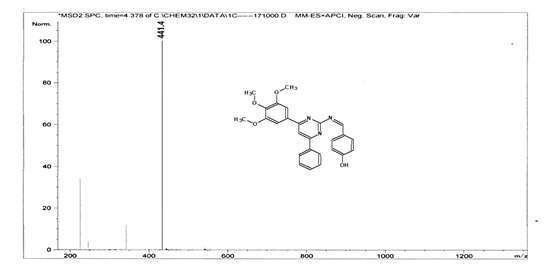
4-[(Z)-{[4-phenyl-6-(3,4,5-trimethoxyphenyl)pyrimidin-2-yl]imino}methyl]phenol 5g. Molecular Formula: C26H23N3O4; Composition: C(70.73%), H(5.25%), N(9.52%), O(14.50%); Yield: 81.01%; M.P: 154℃; 1H NMR (400 MHz, DMSO-d6) δ 9.43 (s, 1H), 8.38 (s, 1H), 8.13 (s, 1H), 8.02 – 7.95 (m, 2H), 7.83 – 7.76 (m, 2H), 7.56 – 7.47 (m, 2H), 7.41 (ddt, J = 7.9, 6.7, 1.5 Hz, 1H), 7.05 (s, 2H), 6.98 – 6.91 (m, 2H), 3.87 (s, 6H), 3.79 (s, 3H); 13C NMR (100 MHz, DMSO-d6) δ 170.84, 165.28, 163.06, 160.63, 156.22, 153.02, 142.01, 136.69, 132.49, 130.19, 128.95, 128.57 (d, J = 2.5 Hz), 126.85, 116.54, 113.19, 108.15, 60.78, 56.26; FT-IR (KBr, v max (cm-1)): 3162 (Ar–CH), 2832 (Alif.–CH), 1278 (C–N), 1659 (C=N), 3625 (O–H), 1033 & 1239 (C–O); ESI-MS: 441.4 [M+H]+.
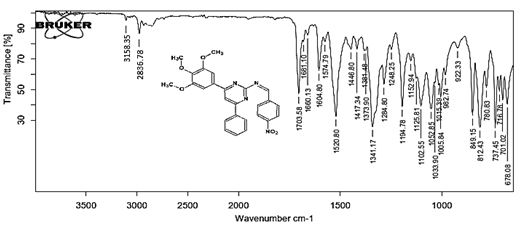
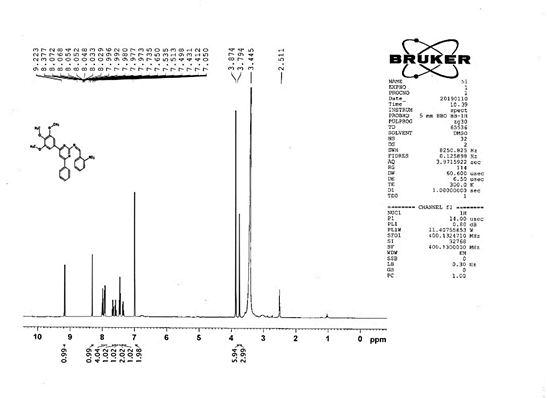
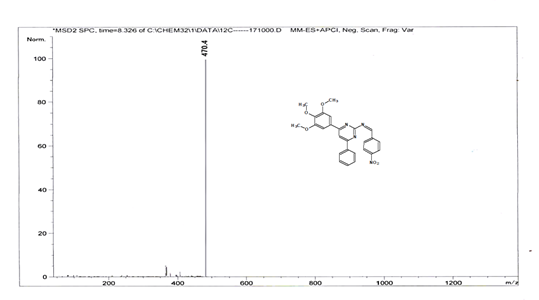
(Z)-1-(4-nitrophenyl)-N-[4-phenyl-6-(3,4,5-trimethoxyphenyl) pyrimidin-2-yl] methanimine 5h. Molecular Formula: C26H22N4O5; Composition: C(66.37%), H(4.71%), N(11.91%), O(17.00%); Yield: 83.17%; M.P: 162℃; 1H NMR (400 MHz, DMSO-d6) δ 9.43 (s, 1H), 8.38 (s, 1H), 8.25 (d, J = 1.2 Hz, 4H), 8.02 – 7.95 (m, 2H), 7.56 – 7.47 (m, 2H), 7.41 (ddt, J = 7.9, 6.7, 1.5 Hz, 1H), 7.05 (s, 2H), 3.87 (s, 6H), 3.79 (s, 3H); 13C NMR (100 MHz, DMSO-d6) δ 170.55, 165.28, 163.06, 160.63, 153.02, 149.30, 142.01, 138.99, 136.69, 130.82, 130.19, 128.95, 128.57 (d, J = 2.5 Hz), 124.60, 113.19, 108.15, 60.78, 56.2; FT-IR (KBr, v max (cm-1)): 3158 (Ar–CH), 2836 (Alif.–CH), 1284 (C–N), 1660 (C=N), 1033 & 1248 (C–O), 1341 & 1520 (N=O); ESI-MS: 470.4 [M+H]+.
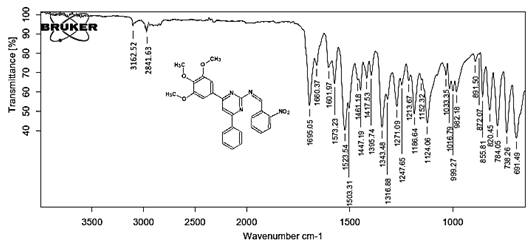
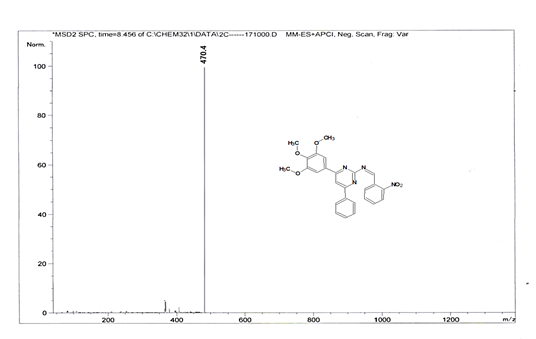
(Z)-1-(2-nitrophenyl)-N-[4-phenyl-6-(3,4,5-trimethoxyphenyl)pyrimidin-2-yl] methanimine 5i. Molecular Formula: C26H22N4O5; Composition: C(66.37%), H(4.71%), N(11.91%), O(17.00%); Yield: 77.65%; M.P: 158℃; 1H NMR (400 MHz, DMSO-d6) δ 9.22 (s, 1H), 8.38 (s, 1H), 8.09 – 7.95 (m, 4H), 7.73 (td, J = 7.4, 1.5 Hz, 1H), 7.65 (td, J = 7.5, 1.6 Hz, 1H), 7.56 – 7.47 (m, 2H), 7.41 (ddt, J = 7.9, 6.7, 1.5 Hz, 1H), 7.05 (s, 2H), 3.87 (s, 6H), 3.79 (s, 3H); 13C NMR (100 MHz, DMSO-d6) δ 165.14, 164.40, 163.06, 160.63, 153.02, 149.70, 142.01, 136.69, 132.02, 131.25, 130.79, 130.19, 128.92 (d, J = 6.1 Hz), 128.57 (d, J = 2.5 Hz), 124.61, 113.19, 108.15, 60.78, 56.26; FT-IR (KBr, v max (cm-1)): 3162 (Ar–CH), 2841 (Alif.–CH), 1271 (C–N), 1660 (C=N), 1033 & 1247 (C–O), 1343 & 1523 (N=O); ESI-MS: 470.4 [M+H]+.
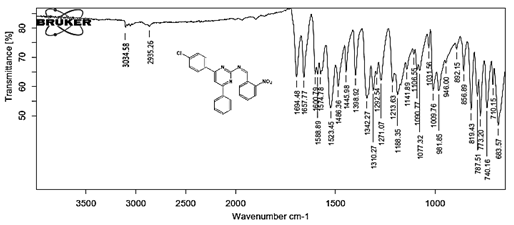
(Z)-1-(2,4-dichlorophenyl)-N-[4-phenyl-6-(3,4,5-trimethoxyphenyl)pyrimidin-2-yl] methanimine 5j. Molecular Formula: C26H21Cl2N3O3; Composition: C(63.17%), H(4.28%), Cl(14.34%), N(8.50%), O(9.71%); Yield: 80.52%; M.P: 168℃; 1H NMR (400 MHz, DMSO-d6) δ 9.07 (s, 1H), 8.38 (s, 1H), 8.02 – 7.92 (m, 3H), 7.70 (d, J = 1.5 Hz, 1H), 7.56 – 7.46 (m, 3H), 7.41 (ddt, J = 7.9, 6.7, 1.5 Hz, 1H), 7.05 (s, 2H), 3.87 (s, 6H), 3.79 (s, 3H); 13C NMR (100 MHz, DMSO-d6) δ 165.73, 164.42, 163.06, 160.63, 153.02, 142.01, 138.44, 136.69, 135.70, 131.25, 131.03, 130.19, 129.44, 128.95, 128.77 – 128.42 (m), 113.19, 108.15, 60.78, 56.26; FT-IR (KBr, v max (cm-1)): 3159 (Ar–CH), 2839 (Alif.–CH), 1278 (C–N), 1659 (C=N), 1033 & 1247 (C–O), 1318 (C–Cl); ESI-MS: 494.3 [M+H]+.
(Z)-1-(4-chlorophenyl)-N-[4-phenyl-6-(3,4,5trimethoxyphenyl)pyrimidin-2-yl] methanimine 5k. Molecular Formula: C26H22ClN3O3; Composition: C(67.90%), H(4.82%), Cl(7.71%), N(9.14%), O(10.44%); Yield: 75.08%; M.P: 144℃; 1H NMR (400 MHz, DMSO-d6) δ 9.43 (s, 1H), 8.38 (s, 1H), 8.02 – 7.91 (m, 4H), 7.73 – 7.66 (m, 2H), 7.56 – 7.47 (m, 2H), 7.41 (ddt, J = 7.9, 6.7, 1.5 Hz, 1H), 7.05 (s, 2H), 3.87 (s, 6H), 3.79 (s, 3H); 13C NMR (100 MHz, DMSO-d6) δ 170.61, 165.28, 163.06, 160.63, 153.02, 142.01, 138.21, 136.69, 132.88, 131.66, 130.19, 129.41, 128.95, 128.57 (d, J = 2.5 Hz), 113.19, 108.15, 60.78, 56.26; FT-IR (KBr, v max (cm-1)): 3163 (Ar–CH), 2857 (Alif.–CH), 1277 (C–N), 1659 (C=N), 1033 & 1248 (C–O), 1318 (C–Cl); ESI-MS: 459.9 [M+H]+.
N,N-dimethyl-4-[(Z)-{[4-phenyl-6-(3,4,5-trimethoxyphenyl)pyrimidin-2-yl]imino} methyl]aniline 5l. Molecular Formula: C28H28N4O3; Composition: C(71.18%), H(6.02%), N(11.96%), O(10.24%); Yield: 82.04%; M.P: 180℃; 1H NMR (400 MHz, DMSO-d6) δ 9.43 (s, 1H), 8.38 (s, 1H), 8.02 – 7.95 (m, 2H), 7.76 – 7.68 (m, 2H), 7.56 – 7.47 (m, 2H), 7.41 (ddt, J = 7.9, 6.7, 1.5 Hz, 1H), 7.05 (s, 2H), 6.74 – 6.67 (m, 2H), 3.87 (s, 6H), 3.79 (s, 3H), 3.00 (s, 6H);13C NMR (100 MHz, DMSO-d6) δ 170.90, 165.28, 163.06, 160.63, 153.02, 151.96, 142.01, 136.69, 131.71, 130.19, 128.95, 128.57 (d, J = 2.5 Hz), 125.75, 113.19, 111.59, 108.15, 60.78, 56.26, 40.27; FT-IR (KBr, v max (cm-1)): 3160 (Ar–CH), 2931 (Alif.–CH), 1278 (C–N), 1658 (C=N), 1033 & 1229 (C–O); ESI-MS: 468.5 [M+H]+.
Computational study
The activity of compounds against CYP450 subtypes (CYP3A4, CYP2D6, CYP2C19, CYP2C9, CYP1A2) was predicted using OCHEM. Bioactivity scores were predicted by online Molinspiration cheminformatics. The drug-likeness and toxicity properties were determined with the OSIRIS property explorer (Data warrior). The human brain permeability and intestinal absorption were forecasted by the construction of a Brain Or IntestinaL EstimateD permeation (BOILED) -Egg model, using the Swiss-ADMET web tool.
Biological evaluation of the synthesized compounds 5a–l
Blood from the healthy human volunteer who has not taken any NSAIDs for two weeks before the experiment was collected and was transferred into the centrifuge tubes which were then centrifuged at 3000 rpm for 10 min. The RBC’s suspension was washed thrice with an equal volume of Normal saline (NS). The volume of blood was measured and reconstituted to 10% v/v suspension with NS.
The mixture consisting of a 1 ml test sample in a concentration range of 50 - 250 μg/ml and 1 ml of 10 % RBCs suspension were taken into the centrifuge tubes. The tubes were incubated for 30 min on a water bath maintained at 560 C which was then cooled under running tap water, the mixture was centrifuged at 2500 rpm for 5 min and the OD values of the supernatant were measured at 660 nm. Instead of a test sample, saline was taken as the control and Aspirin as a reference drug.
The % inhibition of Haemolysis was calculated from the formula:
%Inhibition= (O.D of control-O.D of test)/(O.D of control)
Stable DPPH radical in methanol solution was reacted with the samples (5a-5l) at concentrations of 2, 4, 6, 8, 10 µg/mL The reaction mixture consisting of 2.5 mL of test samples (5a-5l) in methanol and 1 mL of 0.1 mM DPPH solution in methanol were allowed to react at room temperature. After 30 min the absorbance of this solution was noted at 517 nm using a UV-VIS spectrophotometer. The mixture of methanol (1 mL) and standard (Ascorbic acid) (2.5 mL) served as a blank and a mixture of 1 mL DPPH radical solution and methanol (2.5 mL) was used as control. The scavenging activity % (AA %) was determined by
%Inhibition= (O.D of control-O.D of test)/(O.D of control)
IC-50 values were determined from the % Inhibition.
Results and Discussion
The titled compounds i.e. SBs were synthesized in three steps process via the formation of chalcones. Step-1 for chalcone preparation involved solvent-free condition, by frictional force applied through grinding various aldehydes with acetophenone in a mortar and a pestle. [32] The observations showed no difference in yield and purity. This method of preparation of chalcones reduced the cost of solvent, time, and usage of organic solvents such as alcohol. This method has greater reliability in normal laboratory conditions. Step-2 involved the formation of substituted Phenyl pyrimidine-2-amine derivatives by cyclization of chalcones with guanidine hydrochloride at a temperature of 60ᵒC in presence of DMF. DMF is a polar aprotic hydrophilic solvent served as a good catalyst and a reagent. In step-3 The prepared Phenyl pyrimidine-2-amine was condensed with the corresponding aldehyde in ethanol in the presence of sodium hydroxide.
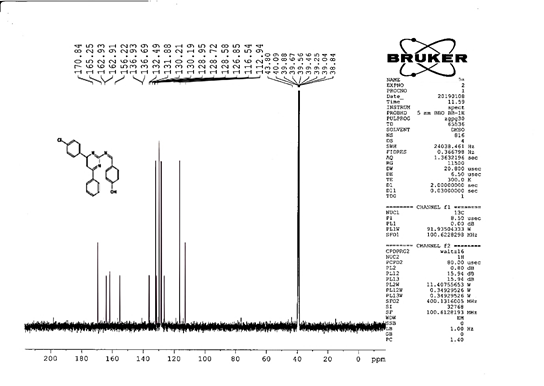
The progress of the reaction and purity of the compounds was assessed by thin-layer chromatography and their melting points. Characterization was done by FTIR, proton and C-13 NMR spectroscopy done on Bruker at 400 and 100 MHZ
The present work also focused on computational tools used to predict the properties, toxicities, and activity of the compounds on various targets.
Cytochrome P-450 and its subtypes are an important set of enzymes involved in the metabolism of various drugs. The present work involves the in-silico study of the synthesized drug candidates by these enzymes.
OCHEM (online chemical modeling), a web-based tool, was chosen to evaluate the activity of the synthesized SB against the cytochromes P-450 (CYPs). OCHEM forecasts the capability of a chemical moiety as an inhibitor or non-inhibitor of CYP-450 subtypes (CYP3A4, CYP2C19, CYP2D6, CYP2C9, CYP1A2). [33] The inhibitory activity of compounds against the cytochromes P450 (CYPs) was depicted in Fig 2. Twenty % of the compounds actively inhibited CYP2D6. The enzyme CYP2C19 was inhibited by 15% of the compounds.
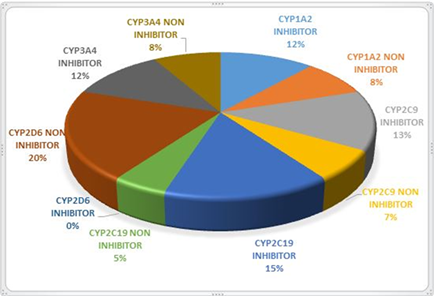
Figure 2: Pie chart representing the activity of Schiff bases against CYP-450 subtypes
Molinspiration cheminformatics was chosen as a web tool to assess the potential biological use of the synthesized SB across numerous therapeutic targets. The bioactivity score prediction for drug targets includes GPCR ligands, ion channel modulators, enzymes, kinase inhibitors, and nuclear receptors. [34] Chemical moieties with a bioactive score lower than -0.50 are taken as less active whereas scores greater than 0.00 exhibit significant potentiality. Scores within the range of -0.50 to 0.00 can be considered reasonably active. The bioactive scores of the synthesized SB were tabulated in Table 2. Compounds 5g, 5l is potentially active and the rest of them are moderately active against kinase. All the compounds are moderately active against the Ion channel modulator, GPCR ligand, Nuclear receptor ligand. Compounds 5a, 5f, 5g, 5l showed moderate score against protease and have prospective for additional structural alteration using QSAR studies.
Table 2: Bioactive score of Schiff Bases against drug targets
|
compounds |
GPCR ligand |
Ion channel modulator |
Kinase inhibitor |
Nuclear receptor ligand |
Protease inhibitor |
Enzyme inhibitor |
|
|
1 |
5a |
-0.24 |
-0.35 |
-0.01 |
-0.19 |
-0.48 |
-0.10 |
|
2 |
5b |
-0.40 |
-0.42 |
-0.16 |
-0.39 |
-0.59 |
-0.24 |
|
3 |
5c |
-0.44 |
-0.43 |
-0.22 |
-0.34 |
-0.60 |
-0.30 |
|
4 |
5d |
-0.30 |
-0.41 |
-0.06 |
-0.36 |
-0.51 |
-0.16 |
|
5 |
5e |
-0.31 |
-0.43 |
-0.07 |
-0.33 |
-0.53 |
-0.18 |
|
6 |
5f |
-0.27 |
-0.39 |
-0.01 |
-0.29 |
-0.50 |
-0.16 |
|
7 |
5g |
-0.26 |
-0.36 |
0.01 |
-0.24 |
-0.47 |
-0.09 |
|
8 |
5h |
-0.39 |
-0.43 |
-0.12 |
-0.39 |
-0.56 |
-0.21 |
|
9 |
5i |
-0.42 |
-0.44 |
-0.17 |
-0.36 |
-0.57 |
-0.26 |
|
10 |
5j |
-0.32 |
-0.42 |
-0.05 |
-0.39 |
-0.53 |
-0.17 |
|
11 |
5k |
-0.30 |
-0.40 |
-0.04 |
-0.35 |
-0.51 |
-0.16 |
|
12 |
5l |
-0.28 |
-0.41 |
0.01 |
-0.31 |
-0.48 |
-0.14 |
The Insilco toxicity properties and drug-likeness were obtained from OSIRIS property explorer. Druglikeness, a qualitative concept used in drug design for how “drug-like” a substance is to bioavailability usually given a score based on the Lipinski rule of five concepts. [35] Compounds 5b and 5j are more likely to be drugs than all other synthesized compounds. All the compounds are non-mutagenic. Compounds 5b and 5j are highly tumorigenic (Fig 3).
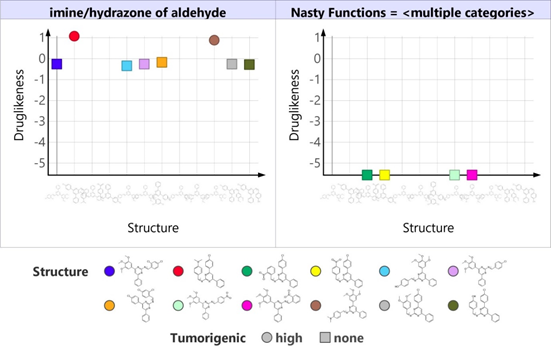
Figure 3: Graph representing drug-likeness, nasty functions & tumorigenicity of Schiff bases(5a-5l)
Human intestinal absorption (HIA) and brain permeability prediction
Brain permeability and HIA are two important pharmacokinetic parameters that contribute to the bioavailability and cell permeability to a considerable degree. To predict the HIA and brain permeability, we constructed a BOILED-Egg model on the online Swiss ADMET tool by the tendering SMILE notations as input [36] which resulted in the creation of the BOILED-Egg model with WLOGP Vs TPSA on the x & y-axis respectively (Fig- 4). Molecules that fall in the yellow region depict the BBB permeation whereas the white eclipse region represents gastrointestinal absorption. Among all the molecules analyzed, Compounds 5a, 5e, 5f, 5g, 5k, 5l were well-absorbed but have no access to the brain. Compounds 5a, 5b, 5c, 5h, 5i were non-substrates for P-GP while the others are subjected to P-GP efflux. Compound 5a has good intestinal absorption but it actively effluxes from the intestine. None of the synthesized compounds can cross the BBB. The conclusions drawn from these studies would be helpful to further improve their pharmacokinetic properties.
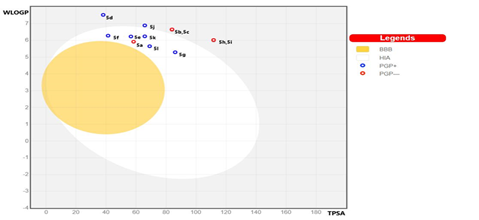
Figure 4: Graphical representation of Brain Or Intestinal Estimated permeation
HIA: Human Intestinal Absorption BBB: Blood-Brain Barrier PGP: Permeability Glyco Protein
Anti-inflammatory activity
The anti-inflammatory action was measured using a heat-induced hemolysis method. The synthesized compounds 5a, 5d, 5g, 5h, 5l showed membrane stabilization activity by inhibiting heat-induced lysis of the erythrocyte membrane. The percentage of membrane stabilization for synthesized compounds and standard were done at 50, 100, 150, 200, 250 µg/ml. Compounds 5a, 5h showed maximum inhibition of 70.53%,69.74% (Table 3) respectively which were closer to that of the standard aspirin (72.91%). From Fig 5 it is evident that the anti-inflammatory activity of the synthesized compounds is completely concentration-dependent.
Table 3: Anti-inflammatory activity
|
S.No |
Compound |
% inhibition |
||||
|
50 µg/ml |
100 µg/ml |
150 µg/ml |
200 µg/ml |
250 µg/ml |
||
|
1 |
5a |
39.32 |
44.94 |
52.19 |
63.31 |
70.53 |
|
2 |
5b |
15.73 |
21.12 |
29.21 |
43.82 |
50.05 |
|
3 |
5c |
24.49 |
26.74 |
33.11 |
40.22 |
49.21 |
|
4 |
5d |
34.05 |
42.07 |
49.38 |
58.34 |
64.87 |
|
5 |
5e |
19.24 |
23.48 |
29.59 |
35.59 |
42.80 |
|
6 |
5f |
25.61 |
27.83 |
30.31 |
34.82 |
40.44 |
|
7 |
5g |
33.23 |
40.28 |
46.42 |
57.23 |
66.58 |
|
8 |
5h |
36.56 |
47.69 |
53.58 |
60.21 |
69.74 |
|
9 |
5i |
26.54 |
32.85 |
42.81 |
49.95 |
52.91 |
|
10 |
5j |
24.58 |
28.34 |
33.86 |
37.29 |
42.18 |
|
11 |
5k |
27.24 |
32.89 |
40.47 |
49.57 |
55.23 |
|
12 |
5l |
34.59 |
48.20 |
53.43 |
61.68 |
67.92 |
|
13 |
Aspirin |
35.29 |
48.83 |
56.75 |
63.27 |
72.91 |
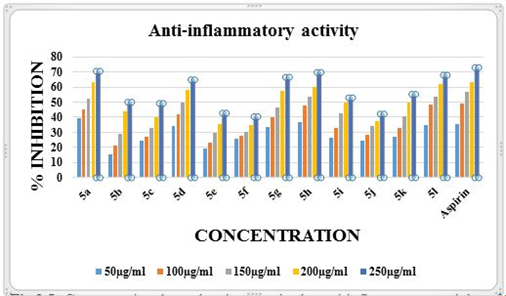
Figure 5: Concentration-dependent increase in the anti-inflammatory activity of Schiff bases
Antioxidant activity
DPPH assay is widely used to evaluate the compounds for their antioxidant activity because of its reliability and simple procedure. The results of the DPPH radical scavenging activity of the compounds are shown in Table 4. The Lower the IC-50 value the better is the DPPH activity. The compounds 5a, 5f, 5i, 5j showed better scavenging activities in DPPH assay. It was important to note that compounds 5j have the IC-50 value closer to that of ascorbic acid (3.03) (Fig 6). The DPPH activity results of the tested compounds have shown a concentration-dependent increase in their antioxidant activity.
Table 4: Antioxidant activity
|
S.No |
Compound |
IC50 |
% inhibition |
||||
|
2 µg/ml |
4 µg/ml |
6 µg/ml |
8 µg/ml |
10 µg/ml |
|||
|
1 |
5a |
5.26 |
35.42 |
39.96 |
47.87 |
53.41 |
59.25 |
|
2 |
5b |
8.68 |
28.43 |
36.21 |
41.14 |
48.62 |
53.54 |
|
3 |
5c |
10.66 |
24.82 |
30.21 |
36.81 |
41.81 |
48.22 |
|
4 |
5d |
9.61 |
27.43 |
34.26 |
40.31 |
44.59 |
51.19 |
|
5 |
5e |
8.94 |
19.82 |
25.80 |
32.96 |
43.57 |
58.23 |
|
6 |
5f |
6.0 |
32.14 |
48.21 |
53.57 |
58.92 |
57.14 |
|
7 |
5g |
8.57 |
23.51 |
32.54 |
39.96 |
47.26 |
55.72 |
|
8 |
5h |
7.66 |
30.41 |
37.44 |
43.80 |
51.62 |
57.92 |
|
9 |
5i |
5.95 |
36.60 |
45.34 |
49.85 |
57.33 |
61.59 |
|
10 |
5j |
4.23 |
41.44 |
52.31 |
55.88 |
59.67 |
63.29 |
|
11 |
5k |
6.92 |
38.59 |
47.83 |
52.51 |
58.26 |
64.11 |
|
12 |
5l |
7.30 |
34.51 |
42.56 |
47.16 |
50.81 |
57.33 |
|
13 |
Ascorbic acid |
3.03 |
43.91 |
56.13 |
59.55 |
62.72 |
67.53 |
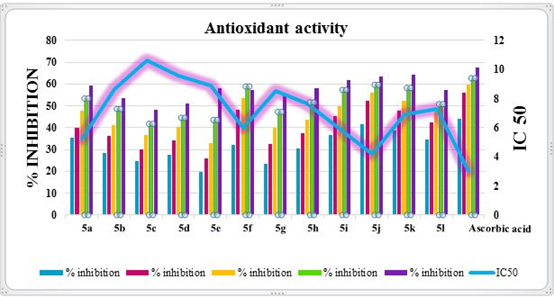
Figure 6: Antioxidant activity of Schiff bases
Conclusion
The present study based on the above results indicated that all the synthesized compounds, pyrimidine SB exhibited antioxidant and anti-inflammatory activities with less toxicity. Among all the 12 titled compounds 5a and 5f were found to greatly influence the activity which may be due to the type of substituents present on the ring. The study carried out considered the three-dimensional nature of chemical structures that played an important part in ligand-receptor binding and assisted in providing an approach for further optimization and development of new leads.
Key Messages
A group of 6-aryl substituted pyrimidine Schiff bases were synthesized by condensation of 6-phenyl pyrimidine 2-amine with different aldehydes. All the synthesized compounds, exhibited antioxidant and anti-inflammatory activities with less toxicity.
Acknowledgement
This research has been funded by Scientific Research Deanship at the University of Ha’il – Saudi Arabia through project number RG-191339.
References