Linum Usitatissimum L. Protects Against Dyslipidemia and Oxidative Damage in Streptozotocin-Induced Diabetic Rats
Fatima Zohra Alachaher 1, Djamil Krouf 1*, Zoheir Mellouk 2, Nawal Taleb-Dida 1
|
|
|
ABSTRACT
Flaxseeds (Linum usitatissimum) (LU) are the richest source of α-linolenic acid and lignans. The present investigation aimed to assess the effect of Linum usitatissimum L. (LU) powder on experimental streptozotocin (STZ)-induced diabetes in Wistar rats. STZ was injected at a single dose of sixty mg/kg of body weight (BW) to induce diabetes mellitus. 20 diabetic rats were allocated into two groups and fed a casein diet supplemented or not with LU powder (1g/100g diet). The animals of the control group (n=10) were fed a standard diet. The animals were sacrificed after 56 days of the experiment and biochemical assays were carried out on plasma and tissue homogenates. Histological modifications were also witnessed in the liver, pancreas, and kidney. At d56, in LU treated vs untreated diabetic groups, glycemia and HBA1c levels were decreased significantly (p<0.05). The body weight was significantly enhanced in LU treated rats as compared to the diabetic group on the 14th day onwards. Our data show that LU improved diabetes-induced dyslipidemia by reducing the levels of total cholesterol (CT) and triacylglycerols (TG) in the liver and plasma. Moreover, LU-treatment lowered significantly plasma phospholipids (PL) and LDL-HDL1-C concentrations in diabetic rats. In reverse, HDL-C concentrations were enhanced in the LU-treated group. Treatment of diabetic rats with LU induced a significant lessening of alanine transaminase (ALT), aspartate transaminase (AST), and alkaline phosphatase (ALP) activities and enhanced renal functions in diabetic rats by decreasing plasma urea, creatinine, and uric acid levels. In addition, LU-treatment normalized thiobarbituric acid reactive substance analysis (TBARS) levels and induced a significant decrease in liver and kidney in diabetic animals. Histopathological observations of the liver, pancreas, and kidney tissues revealed that LU was non-toxic and protected against the deleterious effects of streptozotocin. Our study shows that Linum usitatissimum could prevent diabetic complications by improving diabetes-related dyslipidemia and oxidative damage.
Keywords: Linum usitatissimum, streptozotocin, liver, dyslipidemia, oxidative stress.
Introduction
Diabetes mellitus (DM) is one of the most widespread chronic illnesses worldwide that influences individuals worldwide and is one of the major causes of death [1]. Diabetes is a major danger to global public health, and the numbers of diabetic patients are quickly growing globally [2]. In 2013, the international diabetes federation approximated that 382 million adults had diabetes and 5.1 million deaths happen yearly because of diabetes; the commonness of diabetes has doubled up in the last three decades and is projected to continue rising to 592 million cases by 2035 [3].
DM is a metabolic disorder that is described by nonstandard lipids, hyperglycemia, and protein metabolism resulting from either an absolute or relative deficiency of insulin secretion [4]. DM is often correlated with secondary obstacles like atherosclerosis, hypertension, hypercholesterolemia, myocardial infarction, ischemic attacks, retinopathy, and nephropathy [5]. Numerous investigations have verified the starring role of oxidative stress in diabetes-mediated injuries [6], auto-oxidation of glucose, possibly by oxygen free-radical formation, non-enzymatic protein glycosylation [7, 8], modification in antioxidant enzymes and lipid peroxide formation [9].
Plants are considered as a source of novel pharmaceutical products and inexpensive raw material for the synthesis of some known drugs [10-12], as they have thousands of active secondary metabolites [13]. Numerous medicinal plants have been revealed to exert beneficial impacts on diabetes as some of them may contain insulin-like substances or increase the number of β cells in the pancreas by triggering their regeneration [14, 15]. Plant fiber may also interfere with carbohydrate absorption and lower blood glucose concentrations [16]. Linum usitatissimum (Linn.), usually known as flaxseed or linseed belongs to the Linaceae family. This plant is extensively consumed as a food ingredient [17]. L. usitatissimum (LU) plays a key role in health and ailment as it has α-linolenic acid and secoisolariciresinol diglucoside (SDG) [18]. Several studies indicated the beneficial influences of flaxseed in hypercholesterolemia, diabetes mellitus, and metabolic syndrome (MS) [19-21].
However, there are only a few studies on the antioxidant potential of L. usitatissimum and its association with reactive oxygen species (ROS) production. Hence, considering the traditional use of LU, the present study was conducted to investigate its effects on glycemia and lipemia, and histopathological alterations in the pancreas, liver, and kidney in streptozotocin-induced diabetes in rats.
Materials and Methods
Preparation of Linum usitatissimum
Linum usitatissimum L. was collected from the South-western part of Algeria (Adrar), in February-March 2018. There is almost no rainfall during the year in Adrar, the rainfall averages 71 mm, and the average annual temperature is 28°C. The seeds were identified and authenticated by the Botanical Research Institute of Oran 1 University, Algeria, and grounded to a fine powder utilizing a blender. The resulting flaxseed powder was then deposited in an air-tight container and utilized for further analysis.
Animals and Diets
Male Wistar rats (30) were provided from Animal Research Center, Pasteur Institute, Algiers, Algeria. The rats weighing 267±5g were preserved at stable temperature (22-23°C) and humidity (60%), with 12h light-12h dark cycle (light 07.00-19.00 hours).
The general procedures for the care and utilization of laboratory animals put forward by the Council of European Communities were followed [22] and all the experimental protocols involving the utilization of laboratory animals were verified by the Institutional Animal Ethics Committee of the Nature and Life Sciences Faculty, Oran 1 ABB University. The composition of the diets is depicted in Table 1. Food and water were administered ad libitum.
Induction of diabetes
Induction of diabetes in rats was carried out by a single intraperitoneal injection of recently prepared streptozotocin (STZ) solution (Sigma, St. Louis, MO, USA) dissolved (v/v) in citrate buffer (0.01M, pH 4.5), at a dose of sixty mg/kg body weight (BW). 48 hours after STZ administration, the diabetic state was evaluated by determining glycemia utilizing a glucometer (Accu-Chek® Active, Germany). Only rats with fasting blood glucose levels greater than sixteen mmol/L post-STZ injection were considered diabetic and involved in the investigation [23].
Experimental procedure
Animals were allocated into three groups with ten rats in each (n=10/group) and treated orally once a day for 56 days as follows:
GroupI: Normal healthy control rats fed the standard diet.
Group II: Diabetic rats, fed the standard diet.
GroupIII: Diabetic rats, fed standard diet, containing LU powder (1g/100g diet).
After eight weeks of treatment, the rats were fasted overnight, anesthetized with chloral hydrate 10% (3mg/kg BW), and sacrificed. Blood was gained from the abdominal aorta and collected into tubes comprising sodium ethylenediaminetetraacetic acid (Sigma, St Louis, MO, USA). Blood plasma was obtained by centrifugation at 1000g for 20 minutes at 4°C. The pancreas, liver, and kidney were removed instantly, cleaned with cold saline, and weighed. Aliquots of plasma and 50 to 100 mg of each tissue were deposited at -70°C until examined. A small section of the tissues was fixed with 10% formalin (v/v) for histopathological analysis.
Isolation of plasma lipoproteins
Plasma lipoproteins were isolated by precipitation utilizing MgCl2 and phosphotungstate as described earlier by Burstein et al. [21].
Biochemical measurements
A glucometer (Accu-Chek® active, Germany) was utilized to determine blood glucose at weekly intervals i.e. on days 0, 7, 14, 21, 28, 35, 42, 49 and 56, after the onset of the investigation. The whole blood was collected from the abdominal aorta after blood glucose measurement on day 56. Insulin concentration was measured utilizing an enzymatic immunoassay kit (Spi-Bio, Le Bretonneux, France). Plasma total cholesterol (TC), triacylglycerols (TG), creatinine, urea, and uric acid were determined by enzymatic approaches (kits; Biocon Diagnostik). Phospholipids (PL) were calculated by the enzymatic technique (kit; Cypress Diagnostics). Alanine transaminase (ALT; EC 2.6.1.1), aspartate transaminase, (AST; EC 2.6.1.2), and alkaline phosphate (ALP; EC 3.1.3.1) were determined by enzymatic colorimetric assays (kits; Biocon Diagnostik).
Lipid peroxidation
The Lipid peroxidation was assessed by the complex, formed between malondialdehyde (MDA) and thiobarbituric acid (TBA) [24]. In brief, the kidney and liver samples (0.5 g) were homogenized with 4.5 ml of KCl (1.15%). 100 μl of homogenate was admixed with 750 μl of acetic acid (20%), 100 μl of sodium dodecyl sulfate (8.1%), and 750 μl of TBA reagent (0.8%). The reaction mixture was heated for one hour at 95°C. Then, the tubes were cooled, and 2.5mL of n-butanol-pyridine (15:1) was admixed. The tubes were centrifuged at 4000g for 10 minutes, the upper phase was collected, and absorbance was determined at 532 nm.
Histopathological examination
A small section of the pancreas, liver, and kidney tissues from the experimental animals was fixed in 10% neutral buffered formalin. The standard process of paraffin embedding was utilized to process formalin-deposited tissues; ∼5-mm sections were cut, and stained with hematoxylin and eosin dye. Light microscopy was utilized to assess histological modifications.
Statistical analysis
Findings were expressed as means with their standard errors of 10 rats per group. STATISTICA (version 10; Statsoft, Tulska, Okla) was utilized for statistical analysis. The significance of differences was performed with one-way analysis of variance at a significance level of P<0.05. Further, specific group differences were determined with Dunnett’s honest significant test.
Results
Antidiabetic activity of LU
Food intake, glycemia, and serum insulin concentrations
Food intake was similar in both diabetic groups maintained on standard or experimental diet. A single dose of streptozotocin (60 mg/kg) significantly increased blood glucose concentration in diabetic animals, compared to control rats. LU treatment lowered markedly glycemia (84%) and induced a significant decrease of glycosylated hemoglobin levels in diabetic rats (55%) in diabetic rats (Table 2). Nevertheless, a significant lessening in the level of plasma insulin was witnessed in the untreated diabetic rats contrasted to the normal rats. The level of plasma insulin was further reduced in the untreated diabetic rats after fifty six days. Treatment with LU was revealed to produce the most significant (p <0.05) effect on the level of plasma insulin. It improved the level of plasma insulin virtually to the normal (Table 2).
Effect of LU on glycemia
Figure 1 showed the evolution of blood glucose levels in normal and diabetic rats on 0, 7, 14, 21, 28, 35, 42, and 56 days after LU treatment. In the control group (G1), the rats have consistently maintained their fasting blood glucose levels close to 5.01±0.66 mmol/l. Post-administration of STZ, all diabetic groups had much greater levels of fasting blood glucose. The rats in the diabetic group (G2) had a primary mean fasting blood glucose level of approximately 29.53±1.55 mmol/l. Subsequently, in the diabetic group, these levels were increased to 32.80±1.21 mmol/l on day 56. The elevation of fasting glucose levels in the diabetic group (G2) was statistically significant as compared to the LU group (p< 0.05). Treatment of diabetic rats with flaxseed lessened the upraised levels of fasting blood glucose throughout the study period. In group (G3), the mean fasting blood glucose slightly increased to 27.2±1.01 mmol/l on the initial day, then dropped to 12.03±1.138 mmol/l on day 14, and continuously decreased to reach 8.76±0.78 mmol/l and 5.4± 1.54 mmol/l on days 35 and 56, respectively. The reduction of fasting glucose levels in diabetic rats treated with the flaxseed (G3) was statistically significant as compared to the diabetic group (G2) (p< 0.05) (Figure 1).
Effect of LU on body weight of rats
After eight weeks, a significant reduction in body weight (BW) was observed in diabetic rats as compared to controls. In opposition, BW was greater by 67% in LU-treated diabetic rats than that in diabetic rats, fed standard diet. No significant difference was witnessed in BW between LU-treated and normal diet-fed rats (Figure 2).
Effect of LU on lipid profile
In diabetic rats, there was a significant increase in plasma total cholesterol, and triacylglycerols, as compared to control rats. Liver total cholesterol, triacylglycerols, and phospholipids concentrations were reduced by 26%, 33%, and 22%, respectively in LU-treated animals compared with untreated diabetic rats. In plasma, LU-treatment lowered significantly TC (-44%) and LDL-HDL1-C (-64%) concentrations in diabetic rats. Nonetheless, plasma TG and PL levels were diminished by 49% and 61 %, respectively in the LU-treated group in comparison with the diabetic untreated group. Moreover, TC/HDL-C ratio or LDL-C/HDL-C which are markers of dyslipidemia were significantly reduced in LU treated group than the untreated diabetic group (Table 3).
Effect of LU on Liver and kidney function on lipid peroxidation
Markers of the liver like AST, ALT, ALP enzymatic activities, and bilirubin levels were meaningfully higher in streptozotocin-induced diabetic rats contrasted with the control group. Treatment of diabetic rats with LU induced a noteworthy lessening in liver AST (-42%), ALT (-51%), and ALP (-48%) activities. The bilirubin level was also decreased by 17% in LU-treated diabetic rats (Table 4). Likewise, kidney function markers such as creatinine, plasma urea, and uric acid were meaningfully raised in the streptozotocin-induced diabetic rats as compared to the normal rats. The levels were reduced by 32%, 36%, and 25%, respectively in the LU-treated diabetic group compared to diabetic untreated rats (Table 4).
Thiobarbituric acid reactive substance analysis (TBARS) was carried out to estimate lipid oxidation. TBARS values were increased significantly (P<0.05) in the diabetic group as contrasted with the control group. Conversely, LU-treatment normalized TBARS levels and induced a significant decrease in the liver (-48%) and kidney (-39%) in diabetic animals as compared to diabetic untreated rats (Table 4).
Effect of LU on STZ-induced histopathological alterations
Histology of liver
Photomicrographs of the liver revealed a compact structure, normal hepatic cells with well-preserved cytoplasm, nucleolus, nucleus, and central vein, and no fibrosis in control rats (Fig. 3A). In the case of group 2 diabetic rats, the normal lobular structure was conserved. Nonetheless, the central vein was obviously congested. Focal areas of hemorrhage were also witnessed (Fig. 3B). In group 3 diabetic rats treated with LU, the hepatic portal tracts and central veins seem normal (Figure 3C).
Histology of pancreas
Normal acini and normal cells were witnessed in the islets of Langerhans in the pancreas of the normal control group (Fig. 3D). Extensive damage to islets of Langerhans and decreased dimensions of islets were witnessed in diabetic rats (Figure 3E) while the normal cellular structure was preserved in rats receiving LU treatment (Figure 3F).
Histology of kidney
Histopathological analysis of kidney tissue in normal animals displayed normal structure (Figure 3G). In diabetic rats, mild modifications in the density of mesangial mesangium along with mild thickening of the basement membrane of the arterioles of glomeruli were witnessed (Figure 3H). After treatment with LU, these modifications were improved towards the normal condition. No significant difference was detected in tissue histology in LU treated group as compared to normal control (Figure 3I).
Discussion
In the present study, we have demonstrated the favorable influences of Linum usitatissimum powder in STZ-induced diabetes in rats after 8 weeks of treatment. LU lessened hyperglycemia, lipid peroxidation, and enhanced antioxidant enzyme activity, particularly in liver and kidney in diabetic rats.
STZ, by its toxic influences on pancreatic beta cells, induces diabetes mellitus in experimental animals [25]. Findings of the current investigation indicated that STZ induced diabetes led to a significant rise in serum glucose level and a significant reduction in serum insulin level. Furthermore, STZ resulted in a raise in glycosylated hemoglobin (HbA1c) levels. The diabetogenic influences of STZ established in this investigation are in agreement with Bwititi et al. [26] in STZ-diabetic rats and Serradas et al. [27] who stated that STZ in rats is correlated with hyperglycemia. This diabetogenic influence could be due to the damaging impact of streptozotocin on pancreatic islets. The mechanism of lessened insulin secretion could be attributed to the resultant hyperglycemia that induced anomalies in insulin action and secretion [28]. Hyperglycemia is also associated with the consequences of hyperinsulinemia, insulin resistance, and glucose intolerance in diabetes [29]. The marked enhancement in HbA1c level detected in this study in the diabetic group is in accordance with Vijayaraghavan et al. [30] who stated that streptozotocin-induced diabetic rats indicated a decrease in the hemoglobin level and a concomitant enhancement in HbA1c. This could be because of too much glycosylation of a number of proteins including hemoglobin [31, 32].
Hence, observed hypoglycemia with LU treatment may be due to both pancreatic (enhancement of insulin secretion) and extrapancreatic (peripheral utilization of glucose) mechanisms [33]. Numerous studies have proposed that the antihyperglycemic action of traditional antidiabetic plant may be because of, in part, diminished glucose absorption [34, 35]. Several plant species have been defined as hypoglycaemic such as Opuntia streptacantha, Citrullus colocynthis, Trigonella foenum-graecum, Gymnema sylvestre, Allium sativum, Momordica charantia, Ficus bengalensis, Polygala senega, and Aloe vera [36]. Comparable results were also reported formerly by Moree et al. [37], where treatment with synthetic Secoisolariciresinol diglucoside (SDG) offered noteworthy protection against STZ-induced histological modifications and preserved normal tissue architecture.
Likewise, reduced body weight witnessed in diabetic untreated rats in comparison to control rats indicates that loss of body weight is a result of an excessive breakdown of tissue protein [38]. Treatment with L. usitatissimum enhanced bodyweight to a certain extent, representing that control over muscle wasting resulted from glycemic control. This recommends the hypoglycemic influence of L. usitatissimum in diabetic rats [39].
As diabetes is a metabolic disorder, it is characterized not only by increased glucose concentrations but also by an altered level of lipid profile. Serum lipids are regularly raised in diabetes mellitus and signify a risk factor for coronary heart disease [40, 41]. Diabetic dyslipidemia is marked by high triglycerides, cholesterol, and low-density lipoprotein (LDL) and reduced high-density lipoprotein (HDL) [42]. Our data show that LU improved diabetes-induced dyslipidemia by reducing the levels of total cholesterol (CT) and triacylglycerols (TG) in the liver and plasma. Prasad et al. [43] stated that the lessening in hypercholesterolemic atherosclerosis by flaxseed is because of a reduction in serum TC and LDL-C. These effects were probably due to the existence of SDG, a plant lignan found in flaxseed [44, 45]. Moreover, TC/HDL-C ratio or LDL-C/HDL-C which are markers of dyslipidemia were significantly reduced in LU treated group than the untreated diabetic group and this effect could be attributed to the antioxidant property of this plant. A higher value of these atherogenicity ratios obtained in diabetic rats indicates an elevated atherosclerotic and oxidative damage.
Liver enzymes, i.e. AST, ALT, and ALP activities were also enhanced in diabetic rats. Diabetic complications such as enhanced gluconeogenesis and ketogenesis can be in part because of raised hepatic enzymes [45]. In contrast, these activities were significantly reduced in the LU-treated group, indicating lowered hepatic damage induced by diabetes and may further prevent the leakage of marker enzymes from cell cytoplasm to the blood stream. Thus, LU seems to have protective effects on the liver of diabetic rats.
The previous report of Sinha et al. [46] has suggested that, the hypolipidemic activity may be attributed to inhibition of oxidative stress. In recent times, it has been reported that the oxidation of glucose in diabetes mellitus is the main cause of producing oxidative stress [47]. Our result showed that TBARS levels in the liver were decreased significantly in LU-treated diabetic rats as compared to untreated diabetic rats. These findings demonstrate that LU treatment might protect the tissues against the cytotoxic and oxidative stress produced by streptozotocin. Moreover, the reduction in the lipid peroxidation could be due to the improvement of the glycemic control and antioxidant status [48]. Consistent with the current investigation, there are reports that, by scavenging hydroxyl radical, SDG in flaxseed reduces lipid peroxidation [49, 50].
Linum usitatissimum also enhanced renal functions in diabetic rats by decreasing serum urea, creatinine, and uric acid. Streptozotocin has been revealed to induce free radical generation and cause tissue injury. One factor that has been related to renal and cardiovascular ailment is serum uric acid. It has been indicated that circulating uric acid is an independent forecaster for the development of diabetic nephropathy [51]. In our research, the improved uric acid level was witnessed in the diabetic group. Interestingly, the higher levels of uric acid in diabetic rats were diminished in LU treated group [52]. This is consistent with Abdel Moneim et al. [53] who observed that treatment with flaxseed oil significantly improved kidney function.
Moreover, TBARS levels in kidney were reduced significantly in LU-treated diabetic rats as compared to untreated diabetic rats. Additionally, AL-Okbi et al. [54] stated that flaxseed oil conserved the normal histological structure against diabetes-induced injury in rat kidneys. The pancreas is principally susceptible to the streptozotocin-induced free radical injury. Previous studies have revealed that L. usitatissimum is rich in polyphenols and exerts in vitro antioxidant effect [55, 56].
The findings of the current study displayed that LU treatment prevented hyperglycemia and increased body weight in STZ-induced diabetic rats. It also improved the pancreas, liver, and kidney function, and hyperlipidemia because of diabetes. Moreover, histopathological observations of these tissues were concluded and it was found that LU was non-toxic and protected against the deleterious effects of streptozotocin.
After LU treatment, liver of diabetic rat displaying hepatocytes, portal tracts, and central veins seemed normal. The ultrastructure of the STZ diabetic pancreas showed a considerable reduction in the islet Langerhans and depleted islets. These are in agreement with earlier reports [45-57]. The diabetic rats showed pancreatic islet regeneration. The regenerative effect of the pancreatic cells by Linum usitatissimum via exocrine cells of the pancreas may enlighten the positive effects of these agents on the production of insulin. Oscika et al. [58] proposed that streptozotocin-induced diabetes caused an increase in glomerular protein kinase C activity which played a central role in the development of diabetic nephropathy. The exceptional recovery of renal function expected with the treatment of L. usitatissimum. Comparable results have been witnessed with the treatment of alloxan-induced diabetic rats with Trigonella foenum-graecum seed powder by Thakran et al [59].
The mechanism of the ameliorative influence of flaxseed on the histopathological modifications in STZ-induced diabetic rats is still not clear. Nevertheless, flaxseed is one of the richest sources of the plant-based w-3 fatty acid, alpha-linolenic acid (ALA), and its ingestion may help in avoiding or treating a variety of diabetic obstacles [60].
An experimental study in Zucker diabetic fatty Gmi-fa/fa female rats showed that Secoisolariciresinol diglucoside antioxidants from flaxseed can prevent the development of type 1 diabetes by approximately 71% (90) and type 2 diabetes by 80% [61]. Significant improvements were witnessed in glycemic control in type 2 diabetic patients treated for twelve weeks with lignin supplementation derived from flaxseed [19]. Furthermore, lignans act as antioxidants through the direct scavenging of radicals and by preventing lipid peroxidation.
The role of L. usitatissimum in reversing the diabetic state at the cellular level besides the metabolic normalization further demonstrates its potential as an antidiabetic assert.
Conclusion
It is concluded that daily treatment with flaxseed powder on STZ-induced diabetes in rats increases blood glucose level and histopathological of liver, pancreas, and kidney. The experimental evidence obtained from this investigation specifies that flaxseed represents a candidate alternative treatment to control diabetes mellitus.
Author Contributions
The experimental study, sample collection, data interpretation and manuscript writing, F.Z. A.; the conception of the study, experimental study, and manuscript corrected, D. K.; data interpretation and experimental study, Z. M; sample collection, experimental study, and statistical analysis, N. D.T.
Acknowledgments
This research was supported by the General Directorate for Scientific Research and Technological Development of the Algerian Ministry of Higher Education and Scientific Research.
Ethics approval
The general guidelines for the care and use of laboratory animals recommended by the Council of European Communities were followed and all the experimental protocols involving the use of laboratory animals were approved by the Institutional Animal Ethics Committee of Nature and Life Sciences Faculty, Oran 1 ABB University.
Conflicts of interest
The authors declare no conflict of interest.
References
Table 1: Composition of the experimental diet (g/100 g diet)1.
|
|
Group I |
Group II |
Group III |
|
Casein2 |
20 |
20 |
20 |
|
Corn starch3 |
59 |
59 |
58 |
|
Sunflower oil4 |
5 |
5 |
5 |
|
Sucrose5 |
5 |
5 |
5 |
|
Cellulose2 |
5 |
5 |
5 |
|
Vitamin mix6 |
2 |
2 |
2 |
|
Mineral mix7 |
4 |
4 |
4 |
|
Linum usitatissimum8 |
- |
- |
1 |
The units are as follows:
1Diets were isoenergetic (17.80 kJ/g diet) and given in the powdered form.
2PROLABO (Paris, France).
3ONAB (SidiBelAbbès, Algeria).
4CEVITAL (Béjaïa, Algeria).
5ENASUCRE (Sfisef, Algeria).
6UAR 200 (Villemoisson, 91360). Vitamin mixture provided the followingamounts (mg/kg diet): vitamin A, 39 600 UI; vitamin D3, 5000 UI; vitamin B1, 40; vitamin B2, 30; vitamin B3, 140; vitamin B6, 20; vitamin B7, 300; vitamin B12, 0.1; vitamin C, 1600; vitamin E, 340; vitamin K, 3.80; vitamin PP, 200; choline, 2720; folicacid, 10; paraaminobenzoicacid, 180; biotin, 0.6; and cellulose, qsp, 20 g.
7UAR 205B (Villemoisson, 1360, Epinay/S/Orge, France). Mineral mixture provided the following amounts (mg/kg diet): CaHPO4, 17 200; KCl, 4000; NaCl, 4000; MgO2, 420; MgSO4, 2000; Fe2O3, 120; FeSO4, 7H2O, 200; MnSO4, H2SO4, H2O, 98; CuSO4, 5H2O, 20; ZnSO4, 80; CuSO4, 80; CuSO4, 7H2O; and KI, 0.32.
8Prepared in our laboratory as previously described.
Table 2: Food intake, blood glucose and insulin concentration in control and diabetic rats treated or not with LU (Mean values with their standard errors).
|
|
Group I |
Group II |
Group III |
|
Food intake(g/day/rat) |
28.39±6.07 |
29.88±0.33 |
28.58±2.02 |
|
Glycemia (mmol/l) |
5.01±0.66 |
32.80±1.21‡ |
5.41±1.54* |
|
Insulinemia (pmol/l) |
60.27±4.11 |
44.13±4.10‡ |
61.85±6.22* |
|
Glycosylated hemoglobine (%) |
7.36±0.36 |
12.47±1.07‡ |
5.58±0.32* |
Statistical analysis was carried out utilizing the Dunnet’s honest significant test. The values were significantly different at P < 0.05.
(‡): STZ untreated rats (GII) vs. control (GI)(P < 0.05).
(*): STZ treated rats with LU powder (GIII) vs. STZ untreated rats (GII) (P < 0.05).
Table 3: Effect of LU on liver and plasma lipids in control and diabetic rats treated or not with LU (Mean values with their standard errors).
|
|
Group I |
Group II |
Group III |
|
Liver (µmol/g) |
|
|
|
|
Total cholesterol |
42.32±2.55 |
53.84±1.29‡ |
39.82±1.49* |
|
Triacylglycerols |
35.22±2.21 |
37.46±1.82‡ |
25.24±1.09* |
|
Phospholipids |
25.11±1.02 |
28.33±1.63‡ |
22.11±1.52* |
|
Plasma (mmol/l) |
|
|
|
|
Total cholesterol |
1.21±0.15 |
2.01±0.21‡ |
1.12±0.25* |
|
LDL-HDL1-C |
0.34±0.31 |
0.92±0.31‡ |
0.33±0.20* |
|
Triacylglycerols |
0.33±0.09 |
0.96±0.02‡ |
0.49±0.06* |
|
Phospholipids |
0.45±0.10 |
1.31±0.07‡ |
0.51±0.03* |
|
Atherogenic ratios |
|
|
|
|
TC/HDL |
1.09±0.09 |
1.69±0.07‡ |
1.01±0.06* |
|
LDL-HDL1-C/HDL-C |
0.53±0.10 |
2.13±0.11‡ |
0.58±0.20* |
Statistical analysis was carried out utilizing the Dunnet’s honest significant test. The values were significantly different at P < 0.05.
(‡): STZ untreated rats (GII) vs. control (GI)(P < 0.05).
(*): STZ treated rats with LU powder (GIII) vs. STZ untreated rats (GII)(P < 0.05).
Table 4: Liver and kidney biochemical parameters of control and streptozotocin induced diabetic treated or not with LU powder$ (Mean values with their standard errors).
|
|
Group I |
Group II |
Group III |
|
Liver |
|
|
|
|
Total bilirubin ( µmol/l) |
13.01±1.21 |
15.39±1.46‡ |
12.77±1.11* |
|
Aspartate aminotransferase (IU/L) |
51.23±3.27 |
91.43±3.09‡ |
52.83±2.63* |
|
Alanine aminotransferase (IU/L) |
46.82±3.24 |
86.01±2.47‡ |
42.43±4.12* |
|
Alkaline phosphatase (IU/L) |
103.35±3.43 |
178.32±3.25‡ |
92.07±3.68* |
|
TBARS (nmol/g) |
70.64±7.21 |
199.7±11.20‡ |
103.12±4.91* |
|
Kidney |
|
|
|
|
Urea (mmol/l) |
7.44±0.35 |
8.69±0.58‡ |
5.88±1.20* |
|
Creatinine (mmol/l) |
38.44±2.44 |
64.53±0.34‡ |
41.57±1.99* |
|
Uric acid (mmol/l) |
289.91±1.05 |
376.04±0.59‡ |
280.84±0.41* |
|
TBARS (nmol/g) |
92.01±5.32 |
132.5±6.45‡ |
81.05±5.71* |
Statistical analysis was carried out utilizing the Dunnet’s honest significant test. The values were significantly different at P < 0.05.
(‡): STZ untreated rats (GII) vs. control (GI) (P < 0.05).
(*): STZ treated rats with LU powder (GIII) vs. STZ untreated rats (GII) (P < 0.05).
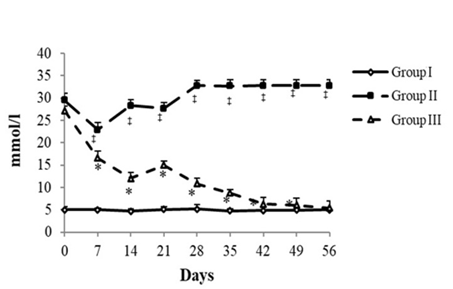
Fig. 1 Glycemia evolution.
Statistical analysis was carried out utilizing the Dunnet’s honest significant test. The values were significantly different at P < 0.05.
(‡): STZ untreated rats (GII) vs. control (GI) (P < 0.05).
(*): STZ treated rats with LU powder (GIII) vs. STZ untreated rats (GII) (P < 0.05).
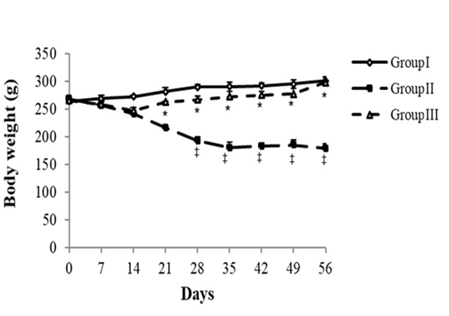
Fig. 2 Changes in body weight.
Statistical analysis was carried out utilizing the Dunnet’s honest significant test. The values were significantly different at P < 0.05.
(‡): STZ untreated rats (GII) vs. control (GI) (P < 0.05).
(*): STZ treated rats with LU powder (GIII) vs. STZ untreated rats (GII) (P < 0.05).
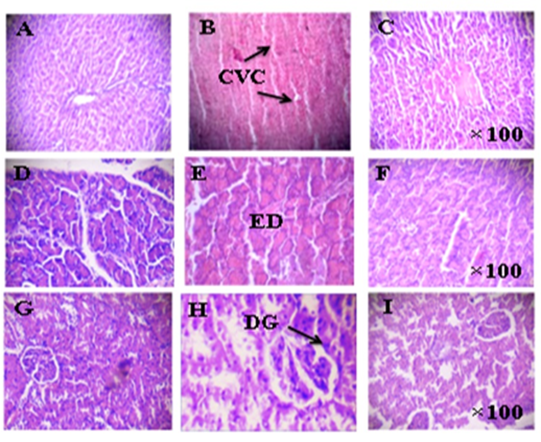
Fig. 3 Histopathological changes in the liver, pancreas, and kidney tissues.
Legends
Liver: (A) Normal control rats, (B) Diabetic rats, (C) Diabetic rats treated with LU
Pancreas: (D) Normal control rats, (E) Diabetic rats, (F) Diabetic rats treated with LU
Kidney: (G) Normal control rats, (H) Diabetic rats, (I) Diabetic rats treated with LU
CVC, central congested vein; ED, extensive damage to islets of Langerhans; DG, degeneration of glomeruli; GS, glomerular swelling
Magnification: H&E X100
