PREVALENCE, RISK FACTORS, AND MONITORING OF AIDS AMONG SYRIANS UNDER THE CIVIL WAR
Gushchina Yu. Sh.1*, Zyryanov S.K. 1, Butranova O.I. 1, Haitham Y. 1, Binenko Elena1, Al Bawareed О.А2
|
|
|
ABSTRACT
According to the United Nations acquired immune deficiency syndrome (AIDS) reports in 2018, the Middle East and North Africa regions are considered as areas of increased concern for human immune deficiency virus (HIV) infection due to high mortality associated with AIDS. This study aims to assess the prevalence of HIV infection among Syrians under the war. The HIV infection was screened among 485,857 Syrian subjects. Also, the antiretroviral drug profile was assessed among HIV positive subjects. The results of HIV-testing using data showed the highest incidence of HIV among Syrians (0.19%). At the end of 2011, the total number of HIV cases on the basis of surveillance started in 1987 was 762, with the figures being 441 (58%) Syrians and 321 (42%) foreigners. Among these HIV-positive individuals, 433 died at the end of 2011 from HIV infection. The geographical distribution of HIV cases in Syria reveals that the majority are found in major cities, with approximately two-thirds (41 percent) of HIV-populated in Damascus and Aleppo (23 percent). The key route for HIV transmission was sexual transmission; 62.8% were heterosexual and 10.5% homosexual. In 2014, there was a higher percentage of mother-to-child HIV transmission during the administration of an injectable medication than was recorded in 2011. Blood transfusions for various diseases is another route of HIV transmission among Syrians. In Syria and, probably, the MENA region, AIDS/HIV has become a serious problem despite the increased availability of free drug therapy. More intensive population screening is required to reduce AIDS/HIV disease distribution dramatically by 2030.
Keywords: HIV infection; AIDS prevention; Mortality; Syria.
Introduction
Human Immunodeficiency Virus (HIV) is now the etiological source of the Acquired Immunodeficiency Syndrome (AIDS), which was first identified as a new disease in 1981 and was then considered a serious global health issue worldwide [1]. AIDS is the final stage of HIV infection, and earlier stages of the virus are asymptomatic [2, 3]. HIV transmission occurs in a variety of ways: Through infected blood and blood products, via sexual contact, and indirectly through the transmission of the virus from infected mother to baby through the placenta, childbirth, or breast feeding [4, 5]. Furthermore, it is characterized in the long term by numerous clinical features and further complications that lack an efficient and effective vaccine. The estimated total world HIV-infected population in 2030 approaches 36.9 million people, with about 1.2 million AIDS-related deaths in 2016 [6, 7].
The total population of the Middle East and North Africa (MENA) region, to its least extent, is estimated to be around 381 million people. This constitutes about 6% of the total world population. The countries that are typically included in MENA are Algeria, Bahrain, Djibouti, Egypt, Iran, Iraq, Israel, Jordan, Kuwait, Lebanon, Libya, Malta, Morocco, Oman, Qatar, Saudi Arabia, Syria, Tunisia, United Arab Emirates, Palestine, and Yemen. Ethiopia and Sudan are also sometimes included in [8].
With 240,000 people reportedly infected with HIV in 2018, the MENA region has the world's lowest incidence, with less than 0.1%. In 2018, around 8400 people died of AIDS-related diseases. This was due to the very limited availability of antiretroviral therapy (ART). Only 32% of all those requiring ART have accessed which this percentage is lower than what is reported globally (59%) [8-10].
An estimated 20000 new HIV-infected subjects were reported across the MENA area in 2018, 4000 more than in 2010 [11]. Most of the HIV-infected subjects were on injectable drugs, sex workers, and gays, all of whom face serious criminalization, discrimination, and stigma [8, 10]
HIV-related death, morbidity, and infectiousness in treated patients have dramatically reduced with antiretroviral therapy (ART). For viral suppression, HIV-infected individuals must receive special care along with anti-HIV drug administration [12, 13].
In October 2014, UNAIDS/WHO 90-90-90 announced that, by 2020, 90% of all HIV-positive people should be aware of their HIV infection, 90% would receive ART and 90% should be fully cured virology. When this three-part target is addressed, at least 73% of all HIV-infected people across the world will be completely suppressed [14-16].
Today, the achievement of these goals of this program is still facing difficulties in European countries and North America, because in the MENA region the majority of the population is below the poverty line compared to the average of world values [8]. Therefore, only 50% of people living with HIV in the MENA region were aware of their HIV status. The gap was too much to achieve 90% of the program; 87 100, ART provided to only 29% of patients with HIV, and viral suppression was achieved in 22% of treated patients [16-18]. These data reflect the complex situation in the whole of the MENA region, and the impact of countries with rather high values of well-being is prominent. Considering HIV and AIDS situation from 2014, there is a complex problem for a state healthcare system, which may result in a rapid rise of new HIV incidences and increase in mortality rates among patients with AIDS [9, 16].
Attempts to estimate the AIDS issue in the Syrian Arab Republic show the Syrian healthcare system's efforts to manage the group of patients even under civil war pressure. Syria descended into a civil war in 2011 following the rebellion of the Arab Spring. In March 2016, the United Nations announced the need for humanitarian aid for 13.5 million Syrians, including 6.6 million internally displaced persons and over 4.8 million refugees outside of Syria. The objective of this study is to estimate the prevalence and profile of the HIV population in the Syrian Arab Republic and to evaluate the ART regimen by its effectiveness under pressure from civil war.
Methods
This study was approved by the Institutional Review Board, Friendship University of Russia, Moscow, Russia. All procedures involving human participants were conducted following the ethical standards of the institutional research committee and with the Helsinki declaration.
This study was conducted in the National AIDS Program, Ministry of Health in Damascus, Syria. The estimation of the number of subjects to be screened was not possible. Data regarding HIV testing were collected from different clinical centers in Damascus, where it is routinely screened in blood donors, and in hospitals where it is mandatory to test HIV before surgery and after counseling and involuntary HIV testing centers.
This study was conducted in Damascus; since it is the capital city of Syria with better medical facilities in comparison with other Syrian cities. Also, the patients attending Damascus hospitals are from different Syrian regions. Therefore, the sample of this study is representative of the Syrian population.
Most of the official epidemiological data on HIV/AIDS in Syria are based on available sources such as routine screening of blood donors, mandatory HIV testing among specific groups of population; female sex workers, gay men and other men who have sex with men, people who inject drugs, and after counseling and voluntary HIV testing centers. In the presence of a positive result, the sample is sent to Damascus for confirmation via the ELISA test or Western blot test. The functioning of this system was interrupted because of war, and legible results are available only from 2009 to 2014.
Results
This study included HIV testing of 485,857 subjects for which demographic data are not available. However, Table 1 show the clinical characteristics of patients who were found to be positive for the diagnosis of AIDS. There were several comorbidities such as tuberculosis, hepatitis C, diabetes, chronic viral infections as well as fungal infections among these patients.
Table 1: Characteristics of HIV-positive patients in the Syrian Arab Republic.
|
Parameter |
Mean (standard deviation) or Number (%), n=204 |
|
Men |
153 (75%) |
|
Women |
51 (25%) |
|
Mean age, years |
41.5 (11.3) |
|
First-line therapy |
172 (84,3 %) |
|
Second-line therapy |
32 (15,7%) |
|
Comorbidities |
|
|
Tuberculosis |
28 (13.7%) |
|
Hepatitis C |
64 (31.4%) |
|
Diabetes mellitus |
3 (1.5%) |
|
Recurrent pneumonia |
7 (4.9%) |
|
Hairy leukoplakia |
3 (1.5%) |
|
Herpetic infection (HV-1) |
15 (7.4%) |
|
Cutaneous Mycosis |
17 (8.3%) |
|
Tracheal and bronchial candidiasis |
3 (1.5%) |
|
Esophageal candidiasis |
3 (1.5%) |
|
Herpes zoster |
2 (1.0%) |
|
Pneumocystis pneumonia |
9 (4.4%) |
|
Toxoplasmosis |
9 (4.4%) |
|
CMV |
5 (2.5%) |
|
HIV-encephalopathy |
5 (2.5%) |
|
Peptic ulcer disease |
3 (1.5%) |
The complications due to immune deficiency depend on the status of immunity as well as on lifestyle and behavior and the presence of hidden infections in the body.
The results related to the testing of blood for HIV infection as well as the HIV incidence in the tested population is given in Table 2.
Table 2: Results of HIV-testing in Syria for 2009-2014 years.
|
Screening Methods for the detection of HIV cases. |
Number of tests |
Percent |
Number of identified HIV cases |
Percent |
HIV incidence in the tested population |
|
Routine screening of blood donors |
412,073
|
84.8% |
2 |
2.9% |
0,0005% |
|
Mandatory HIV testing |
71,648 |
14.7% |
64 |
91.4% |
0.09% |
|
2,089 |
0.4% |
4 |
5.7% |
0.19% |
|
|
Total |
485,857 |
100% |
70 |
100% |
0.014% |
Reliable statistics on HIV incidence dynamics are available from 1987 to 2011 and indicate that HIV infection or AIDS is common. At the end of 2011, the total number of registered HIV cases was 762 including 441 (58%) Syrians and 321 (42%) foreigners. Of these HIV-positive subjects, 433 subjects died at the end of 2011, as shown in figure 1.
The geographical distribution of HIV cases in Syria indicates that the majority of people living with HIV are located in large cities, with almost two-thirds of people living with HIV in Damascus (41%), and Aleppo (23%). About 10 % of people living with HIV were found in Homs, and 5 % were found in As-Suwayda.
Figure 1: The annual incidence of new HIV cases (1987-2011).
This pharmaco-epidemiologic study includes the total number of reported cases and routes of HIV transmission in Syria from 1987 to 2014, according to the data of the National AIDS Program, Ministry of Health, Damascus, Syria. The findings revealed that the leading route is sexual transmission (62.8%, heterosexual, and 10.5%, homosexual) (Table 3).
Table 3: The status of HIV transmission in Syrians from 1987 to 2014, in the Syrian Arab Republic
|
Route of HIV transmission |
Men |
The rate of transmission among men |
Women |
The level of HIV transmission among women |
Total |
Percent |
|
Heterosexual contact |
169 |
60.1% |
64 |
71.1% |
233 |
62.8% |
|
Gay men and other men who have sex with men (MSM) |
39 |
13.9% |
- |
0% |
39 |
10.5% |
|
Blood transfusion |
22 |
7.8% |
9 |
10% |
31 |
8.4% |
|
Injecting drug use |
18 |
6.4% |
0 |
0 |
18 |
4.85% |
|
Mother-to-child transmission |
7 |
2.5% |
11 |
12.2% |
18 |
4.85% |
|
Unknown |
26 |
9.3% |
6 |
6.7% |
32 |
8.6% |
|
Total |
281 |
100% |
90 |
100% |
371 |
100% |
Pharmacotherapy of HIV Infection.
Despite the military action, the Ministry of Health is working with HIV-positive patients in the frame of the National AIDS Program. There is a list of antiretroviral agents provided free of cost by the state as given in Table 4.
Table 4: Antiretroviral agents guaranteed for HIV/AIDS patients in Syria (2018).
|
Trade name of the drug |
Active ingredients |
Dose |
Number of Tablets |
Frequency |
|
Viraday |
TENOFOVIR + EMTRICITABINE + EFAVIRENZ |
300 mg
600 mg |
30 tablets |
Onetabletperday |
|
Duovir-N |
LAMIVUDINE + ZIDOVUDINE + NEVIRAPINE |
150mg
300 mg
200 mg |
60 tablets |
One tablet every 12 hours |
|
KALETRA |
LOPINAVIR |
200 mg/50g |
120 tablets |
Two tablets every 12 hours |
|
DUOVIR |
LAMIVUDINE + ZIDOVUDINE |
150 mg
300 mg |
60 tablets |
One tablet every 12 hours |
|
TRUVDA |
TENOFOVIR + EMTRICITABINE |
300 mg
200 mg |
30 tablets |
One tablet every day |
Generally, the list of drugs for ART presented in Syria reflects the modern single-pill regimen approach, which is according to guidelines for the use of antiretroviral agents [19]. To estimate the actual HIV situation in Syria, we studied data of the National AIDS Program, Ministry of Health, Damascus, Syria, which was relevant for the 2016 year. These data included records of 204 patients diagnosed with HIV and receiving treatment of which 75% (153) were male. There was next distribution according to the year of treatment start in the studied population: 1999 – 1, 2003 – 6, 2004 – 9, 2005 – 7, 2006 – 10, 2007 – 8, 2008 – 15, 2009 – 20, 2010 – 22, 2011 – 35, 2012 – 32, 2013 – 15, 2014 – 15, 2015 – 9.
Diagnostics: CD8 number was performed for 83 patients (37.3%) – test was performed more than once), CD4 – for 135 (79 (58.5%) – test was performed more than once). According to the data of the last testing on CD8, the majority of the subjects (62.7%). the mean value of CD8 was 856.7, and the mean value of CD4 was 449.6 as shown in figure 2 and table 5.
Table 5: Immunological testing in patients with AIDS
|
CD4 cells number (N=135) |
CD8 cells number (N=83) |
|
|
10 to 1954 |
23 to 2577 |
Range |
|
387 |
770 |
Median |
|
449.6(323.0) |
856.7(510.6) |
Mean (Standard deviation) |
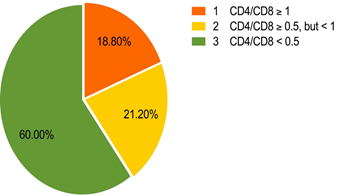
Figure 2: Distribution of CD4/CD8 ratio values in the studied population.
Viral load was determined in 40 subjects but it was done multiple times in only 5 subjects. Of these, only 1 case revealed positive dynamics with an increase in viral load, but there was a decline of about 7 times, in other cases. In the group (8 subjects) with a single test of viral load, the result was negative. However, among the population with a defined viral load, the median was 22308 (Table 6).
Table 6: Viral load among patients with AIDS.
|
|
Viral load (n=40) |
|
Minimum |
395 |
|
Maximum |
31229055 |
|
Median |
22308 |
|
Mean value (Standard deviation) |
1476610,4(6005539.2) |
Discussion
The prevalence of HIV has been documented in various countries worldwide. However, no research has been carried out on HIV infection in Syria during the civil war. This research is the first report on the incidence of AIDS in Syrians and the results showed that HIV or AIDS is widespread. At the end of 2011, there were a total of 762 reported HIV cases, including 441 (58%) Syrians and 321 (42%) foreigners. Of these HIV positive subjects, 433 people died at the end of 2011. In Syria, the regional distribution of cases of HIV shows that most people with HIV are in major cities such as Damascus (41%) and Aleppo (23%) (Tables 2 & 3).
In the MENA region, the number of persons living with HIV per 1000 people (as published by UNAIDS) is provided in figure 1 at the end of 2017 in Algeria – 14000 (0.03 per1.000), Bahrain < 500, Djibouti – 9100 (0.61 per1.000). Egypt – 16.000 (0.02 per1.000), the Islamic Republic of Iran – 600. There are no data on other MENA countries for various reasons; the situation in some of these countries could in the future lead to an explosion of HIV in the MENA region. The Syrian Arab Republic has a wide territory and is eighth amongst other countries in the MENA region with over 18.5 million inhabitants. Military incidents have broken all aspects of public life and have contributed to a health crisis. In 2011, the Syrian Arab Republic was one of six MENA countries that reported 3-month HIV diagnosis data on CD4 counts in newly reported HIV cases at the time of diagnosis. However, the number of cases was very low, 6%. [20].
While HIV/AIDS is a leading cause of disease burden in sub-Saharan Africa, existing evidence indicates that there is significant local variation in HIV prevalence [21]. Sub-national variations in a high spatial resolution across the continent have not been properly studied. This fine estimate of HIV prevalence over time provides an important tool to overcome precisely the interventions needed to control HIV infections in sub-Saharan Africa [21]. In the MENA region, in particular in Syria, such a detailed data analysis is also required.
Comparing with the data available for 2011, there are some changes in the status of routes of HIV transmission. There is an increase in the role of homosexual transmission detected in 2014 (4.85%). Mother-to-child transmission also showed an increase, compared to 0 in 2011, which was similar to an increase via injectable drug administration (4.85% versus 0 in 2011). HIV prevalence among people who inject drugs was previously estimated in Syria in 2006, in a sample of 204 subjects with 96% male. The prevalence of HIV was 0.5% (95% CI: 0.1-2.7) [Syria Mental Health Directorate, Syria National AIDS Programme (2008) in an assessment of HIV risk and seroprevalence among drug users in greater Damascus. Damascus: Syrian Ministry of Health. UNODC. UNAIDS [22]. For the last 5 years, there is no legible data on the HIV situation in the country. The state monitoring system cannot cover regions with active war events and migration of population makes it difficult to manage HIV infection.
ART is recommended to minimize morbidity and mortality associated with HIV infection for all persons with HIV irrespective of their cell count of CD4. According to the recommendation of Panel, the anti-retroviral treatment inhibition regime in a patient who is in need of treatment is usually composed of two nucleoside reverse transcriptase (NRTI) components in conjunction with a third ARV active inhibitor of one of three medications classes: an Integration Beach Transfer Inhibitor (INSTI). Regimes of 2 NRTIs plus PI or NNRTI result in a potent suppression of the virology (< 400 copies ml-1). This can lead to a significant reduction in morbidity and mortality, as shown in other studies [23]. Evaluation of the list of drugs used for HIV/AIDS patients in Syria reveals general accordance with recommendations what groups should be used, but considering certain drug names we can find a difference with the modern antiretroviral drugs of choice. Recommended drugs for initial ART according to updated Guidelines include the following agents.
Bictegravir, Elvitegravir/Cobicistat, Dolutegravir, and Doravirine are not available for patients as free drugs, due to economic reasons.
The world appears unparalleled in ending AIDS since HIV/AIDS remains a leading cause in sub-Saharan Africa for disease and mortality [21, 24, 25]. In the last four decades, clinical advances have seen the once lethal disease successfully treated with lifelong antiretroviral therapy (ART) [25]. While the availability and increased use of TAR have increased rapidly since the mid-2000s, as well as death rates, 34 percent of East and South African citizens and 60 percent of West and Central Africans living with HIV receive no care at all today. [24-26].
The global burden of disease research showed an increase in global health expenditure from 1995 to 2015 [24]. There are currently 1.1 million HIV-positive individuals in the United States, and over 700,000 people have died of AIDS since they first registered in 1981 [27]. 38,281 new cases of HIV were diagnosed in 2017; 81% were men and 19% were women [28]. The degree to which PrEP with oral tenofovir disoproxil fumarate-based treatment reduces the risk of acquiring HIV infection in high-risk individuals is important [28].
In the early stages of infection, the diagnosis of HIV infection is difficult and the transmission is highly successful. The treatment as a preventive technique often compromises this constraint. In addition, HIV testing or receiving treatment is not always easy for most people. However, the detection of cases and HIV care continue to be hampered by ignorance, fear, stigma, homophobia, and other adverse social powers. The US government has announced that it will end the HIV epidemic in the United States in the next ten years [29, 30]. The main target justified by the PARTNER2 results is to diagnose and treat HIV infection rapidly [31]. In MENA regions, for HIV/AIDS prevention, a similar strategy with ample funding is required to follow the above program. [25, 26]. The MDG 6 (Combating AIDS, malaria, and other diseases) was the goal of stopping this outbreak by 2015 and has begun to reverse the spread of HIV/AIDS.
Conclusion
In Syria and, probably, the MENA region, AIDS/HIV has become a serious problem despite the increased availability of free drug therapy. More intensive population screening is required to reduce AIDS/HIV disease distribution dramatically by 2030.
Conflict of interest has not been declared by the authors.
References
1. Ahmed SI, Sulaiman SA, Hassali MA, Farooqui M, Thiruchelvam K, Lee CK. A qualitative evaluation of patients' understanding, expectations and experiences with HIV/AIDS treatment. Archives of Pharmacy Practice. 2019 Oct 1;10(4).
2. Saha M, Bhattacharya S. A Brief Overview on HIV Infection, Diagnosis and Treatment. Current Topics in Medicinal Chemistry. 2019 Nov 1;19(30):2739-41.
3. Zhytnik T, Yermak H, Moskalyova L, Podplota S, Fedorova O, Liapunova V. Socio-Psychological Aspects of HIV Infection Prevention among First-Year University Students as a Health-Saving Factor. International Journal of Pharmaceutical Research & Allied Sciences. 2020;8(1):1-8.
4. Pinnamaneni R, Tanneru P, Vaddavalli N, Tatineni J, Tharani R. In silico Drug interaction studies on HIV Integrase. Journal of Biochemical Technology. 2019;10(3):34.
5. Alsamarrai AS, Abdulla NH, Aldoori MK. Synthesis and Characterization of 2-((4R, 4aR, 5aS, 6S)-1, 3-dioxo-3, 3a, 4, 4a, 5, 5a, 6, 6a-octahydro-4, 6-ethenocyclopropa [f] isoindol-2 (1H)-yl)-N-(Substituted Phenyl) Acetamides Derivatives Anticipated to Inhibit HIV-1 Activity. International Journal of Pharmaceutical and Phytopharmacological Research. 2018 Oct 1;8(5):7-11.
6. Ford N, Ball A, Baggaley R, Vitoria M, Low-Beer D, Penazzato M, Vojnov L, Bertagnolio S, Habiyambere V, Doherty M, Hirnschall G. The WHO public health approach to HIV treatment and care: looking back and looking ahead. The Lancet Infectious Diseases. 2018 Mar 1;18(3):e76-86.
7. Rana S. Knowledge, attitude and practices of female sex workers about HIV/AIDS prevention in Lahore, Pakistan. Journal of Perceptions in Public Health. 2017;1(2):148-56.
8. Mumtaz GR, Hilmi N, Majed EZ, Abu-Raddad LJ. Characterising HIV/AIDS knowledge and attitudes in the Middle East and North Africa: Systematic review and data synthesis. Global Public Health. 2020 Feb 1;15(2):275-98.
9. Hamarsheh O. HIV/AIDS in Palestine: A growing concern. International Journal of Infectious Diseases. 2020 Jan 1;90:18-20.
10. Elgalib A, Shah S, Al-Wahaibi A, Al-Habsi Z, Al-Fouri M, Lau R, Al-Kindi H, Al-Rawahi B, Al-Abri S. The Epidemiology of HIV in Oman, 1984–2018: A Nationwide Study from the Middle East. Journal of Epidemiology and Global Health. 2020 Jan.
11. Gheibi Z, Shayan Z, Joulaei H, Fararouei M, Beheshti S, Shokoohi M. Determinants of AIDS and non-AIDS related mortality among people living with HIV in Shiraz, southern Iran: a 20-year retrospective follow-up study. BMC Infectious Diseases. 2019 Dec;19(1):1-1.
12. Zhu J, Rozada I, David J, Moore DM, Guillemi SA, Barrios R, Montaner JS, Lima VD. The potential impact of initiating antiretroviral therapy with integrase inhibitors on HIV transmission risk in British Columbia, Canada. EClinicalMedicine. 2019 Aug 1;13:101-11.
13. Zicari S, Sessa L, Cotugno N, Ruggiero A, Morrocchi E, Concato C, Rocca S, Zangari P, Manno EC, Palma P. Immune activation, inflammation, and non-AIDS co-morbidities in HIV-infected patients under long-term ART. Viruses. 2019 Mar;11(3):200..
14. Levi J, Raymond A, Pozniak A, Vernazza P, Kohler P, Hill A. Can the UNAIDS 90-90-90 target be achieved? A systematic analysis of national HIV treatment cascades. BMJ global health. 2016 Sep 1;1(2).
15. Sidibé M, Loures L, Samb B. The UNAIDS 90–90–90 target: a clear choice for ending AIDS and for sustainable health and development. Journal of the International AIDS Society. 2016;19(1).
16. UNAIDS (UNAIDS Geneva, 2018).
17. Nasirian M, Karamouzian M, Haghdoost AA. Why is the number of HIV/AIDS-related publications low in the MENA region?. Sexually transmitted infections. 2013 Nov 1;89(Suppl 3):iii10-.
18. Gökengin D, Doroudi F, Tohme J, Collins B, Madani N. HIV/AIDS: trends in the Middle East and North Africa region. International Journal of Infectious Diseases. 2016 Mar 1;44:66-73.
19. Cohn SE. Women in HIV trials: improving adherence and outcomes. The Lancet HIV. 2017 Dec 1;4(12):e530-1.
20. Bozicevic I, Riedner G, Haghdoost A. HIV case reporting in the countries of North Africa and the Middle East. Journal of the International AIDS Society. 2014 Jan;17(1):18962.
21. Dwyer-Lindgren L, Cork MA, Sligar A, Steuben KM, Wilson KF, Provost NR, Mayala BK, VanderHeide JD, Collison ML, Hall JB, Biehl MH. Mapping HIV prevalence in sub-Saharan Africa between 2000 and 2017. Nature. 2019 Jun;570(7760):189-93.
22. Adejumo OA, Malee KM, Ryscavage P, Hunter SJ, Taiwo BO. Contemporary issues on the epidemiology and antiretroviral adherence of HIV‐infected adolescents in sub‐Saharan Africa: a narrative review. Journal of the International AIDS Society. 2015 Jan;18(1):20049.
23. Tseng A, Seet J, Phillips EJ. The evolution of three decades of antiretroviral therapy: challenges, triumphs and the promise of the future. British journal of clinical pharmacology. 2015 Feb 1;79(2):182-94.
24. Dieleman JL, Haakenstad A, Micah A, Moses M, Abbafati C, Acharya P, Adhikari TB, Adou AK, Kiadaliri AA, Alam K, Alizadeh-Navaei R. Spending on health and HIV/AIDS: domestic health spending and development assistance in 188 countries, 1995–2015. The lancet. 2018 May 5;391(10132):1799-829.
25. Anderson SJ, Cherutich P, Kilonzo N, Cremin I, Fecht D, Kimanga D, Harper M, Masha RL, Ngongo PB, Maina W, Dybul M. Maximising the effect of combination HIV prevention through prioritisation of the people and places in greatest need: a modelling study. The Lancet. 2014 Jul 19;384(9939):249-56.
26. McGillen JB, Anderson SJ, Dybul MR, Hallett TB. Optimum resource allocation to reduce HIV incidence across sub-Saharan Africa: a mathematical modelling study. The lancet HIV. 2016 Sep 1;3(9):e441-8.
27. Owens DK, Davidson KW, Krist AH, Barry MJ, Cabana M, Caughey AB, Curry SJ, Doubeni CA, Epling JW, Kubik M, Landefeld CS. Preexposure prophylaxis for the prevention of HIV infection: US Preventive Services Task Force recommendation statement. Jama. 2019 Jun 11;321(22):2203-13.
28. Cohen MS. Successful treatment of HIV eliminates sexual transmission. The Lancet. 2019 Jun 15;393(10189):2366-7.
29. Fauci AS, Redfield RR, Sigounas G, Weahkee MD, Giroir BP. Ending the HIV epidemic: a plan for the United States. Jama. 2019 Mar 5;321(9):844-5.
30. Ratmann O, Van Sighem A, Bezemer D, Gavryushkina A, Jurriaans S, Wensing A, De Wolf F, Reiss P, Fraser C. Sources of HIV infection among men having sex with men and implications for prevention. Science translational medicine. 2016 Jan 6;8(320):320ra2-.
31. Rodger AJ, Cambiano V, Bruun T, Vernazza P, Collins S, Van Lunzen J, Corbelli GM, Estrada V, Geretti AM, Beloukas A, Asboe D. Sexual activity without condoms and risk of HIV transmission in serodifferent couples when the HIV-positive partner is using suppressive antiretroviral therapy. Jama. 2016 Jul 12;316(2):171-81.
