TERATOGENICITY AND ACUTE TOXICITY OF SELECTED PHILIPPINE INDIGENOUS SPICES USING BRINE SHRIMP LETHALITY ASSAY AND ZEBRAFISH ASSAY
Carl Raymond D. Consuegra1*, Felix Michelle B. Custodio1, Jesse Paolo L. Pananganan1, Wendie Jean D. Ramirez1, Jake Joshua C. Garces1,2, Jay P. Picardal1,2
|
|
|
ABSTRACT
Introduction: In the Philippines, spices such as Cinnamomum mindanaense (CM), Illicium verum (IV), and Ocimum spp. (OS) are used for culinary purposes and in traditional herbal medicine practice. These spices have claimed health benefits, but toxicity evaluation of their bioactive constituents have not been thoroughly explored. This study aimed to evaluate the toxicity and teratogenicity of these selected indigenous spices. Materials and Methods: The toxicity of these spices was evaluated using Brine Shrimp Lethality Assay (BSLA, LC50) and Zebrafish Assay (hatchability, morphological abnormalities, and mortality) under a Complete Randomized Design. Results and Discussion: The results in BSLA revealed that the LC50 of CM leaf extract (6.961 μg/ml), IV leaf extract (10.434 μg/ml) and OS leaf extract (15.737 μg/ml) were considered highly toxic, following a linear dose-response trend. Evidence revealed that there was a significant difference (p ≤ 0.05) in the toxicity and teratogenicity of the selected indigenous spices against zebrafish embryo/larvae as evidenced by less hatchability, morphological abnormalities, and high mortality. The LC50 value of CM leaf extract (230.497 μg/ml) and IV leaf extract (278.328 μg/ml) registered medium toxicity. Conclusion: This study provided additional evidence on the suspected toxicities of some indigenous spices when consumed at higher concentrations. Additional studies using other animal models and chronic testing through longitudinal time consideration are needed to further elucidate the consistency of its effect across different vertebrate models.
Keywords: Cinnamomum mindanaense, Illicium verum, Ocimum spp., Brine shrimp lethality assay (BSLA), Zebrafish assay
Introduction
Spices are traditionally used for cooking because of its strong flavors and aroma. [1, 2] Spices are acknowledged to have health benefits to humans but its side effects are less known. [3, 4] Previous studies suggest that spices and their chemical constituents may have toxicological activities. [5, 6] This result in various side effects that differ from its allergic reactions, depending on the level of its consumption and can result in complications in the body. [1]
These complications may affect pregnant women, increasing the risks of birth defects. It is also linked to some speculation that a high risk of congenital anomalies is also caused by an environmental factor. [7] Experts believe that this kind of case includes maternal exposure to harmful products known as teratogens. [8] There is a direct effect on fetal development if the level of toxins and environmental pollutants are very high and there are ample reports on the toxic effects of some spices and herbs in the pediatric population. [5, 7] Exposure to certain toxins, chemicals, medications, alcohol, and radiation during pregnancy may contribute to birth defects. [9]
Every health institution is recommended to construct an accessible emergency wing in its respective facilities [10-12]. Birth defects are malformations or any anomaly that exists since birth. [13] The World Health Organization estimated that 303,000 babies die within four weeks of birth every year. Globally, it has mainly affected the low- and middle-class countries including the Philippines. [9, 13] Birth defects are rampant to some parts of the world, yet health care resources are limited [14]. [15] It can affect any part of the body which can be mild to severe, some can be amended through surgeries and other medications, but it can also be life-threatening and those who survive often undergo lifetime disability. [16] The occurrence of birth defects happens specifically in the first three months of pregnancy. [17] The WHO articulated that approximately 50% of all the congenital defects are likely to be linked to a specific factor such as environmental lifestyle which poses risk to birth defects. [18]
In the record of the registration of birth defects in the Philippines, congenital anomalies rank as the third leading cause of death in the infancy period. [19] After a decade, a retrospective study was conducted and the result shows that birth defects are still the common reason and contribute to the infant and child morbidity in the country. [20] Dr. Delfin Cuajungco said that babies with congenital anomalies die because of complications. [21] The most usual birth defects are malformation of the tongue, mouth, pharynx, cleft palate with cleft lip digestive, cardiovascular, nervous system, and genital organ anomalies. [22, 23] One reason for these morphological abnormalities is the exposure to a certain environmental factor such as toxins and teratogens as well as the presence of heavy metals in herbs and spices. [5, 24, 25]
A collaborative study conducted by the Center for Disease Control and Prevention (CDCP), March of Dimes, and Research Triangle Institute (RTI) International revealed the lack of information on the medicine to use and food to eat during pregnancy. [26] Women tend to categorize medications and other food (vegetables, fruits, and spices) as safe to take but the study shows that this belief results in a riskier problem. [26] Pregnant women should be keen and careful about what she ingests since edible plants like spices contain phytochemicals or substances that if ingested in considerable amounts may affect the pregnancy and cause convolution like malformations or even spontaneous abortions. [27] This imposes the importance of toxicity evaluation (i.e. to characterize the possible toxic effects it can cause to humans). [28]
There are only a few studies conducted about the teratogenic potential and toxicity level of spices (Cinnamomum mindanaense, Illicium verum, and Ocimum spp.). Although these selected indigenous spices have a wide range of uses, there is still a dearth of knowledge on this matter; hence, further investigation about its harmful effects on human development and health is necessary. This study aims to identify the gap of the past study about teratogenicity and toxicity of the selected indigenous spices, in terms of its lethal concentration and morphological endpoints.
Materials and Method:
Plant collection and aqueous extraction
Five hundred grams of mature C. mindanaense, I. verum, and Ocimum spp. leaves were collected separately from the mountain districts of Argao and Alcoy, Cebu. The leaves were washed with distilled water and air-dried for three days after collection. The petiole and midrib of the leaves were removed, leaving only its blade which was shredded and homogenized using an electric blender to obtain a fine powder of the plant samples. For plant extraction, the method of [29] was adopted with modification, as described briefly: for each spice, 11 g powdered leaves (one gram for BSLA; ten grams for Zebrafish Assay) was mixed with 1000 mL of distilled water, boiled for two hours and cooled at room temperature. Cooled aqueous extracts were individually placed in an airtight container, covered with aluminum foil, and stored at 25+1°C until use.
Growth requirement of experimental organisms
Dormant eggs (i.e. freeze-dried cysts) of brine shrimp (Artemia salina) were purchased from a local distributor. The eggs were immersed in the conditioned water until it hatches. Environmental parameters and photoperiod were observed daily throughout the entire incubation period, as follows: temperature (24℃ –28℃
–28℃ ), pH (7.5–8.5), and salinity (25 ppt). [30] Sexually mature zebrafishes (Danio rerio), ~3 months old, 4 cm in length [31, 32] were collected from a local distributor. The fishes were fed twice daily to remain in good health condition [33, 34] and were exposed to a photoperiod of 12h light and the 12h dark cycle. Required physicochemical parameters were maintained and monitored daily, as follows: temperature (28℃
), pH (7.5–8.5), and salinity (25 ppt). [30] Sexually mature zebrafishes (Danio rerio), ~3 months old, 4 cm in length [31, 32] were collected from a local distributor. The fishes were fed twice daily to remain in good health condition [33, 34] and were exposed to a photoperiod of 12h light and the 12h dark cycle. Required physicochemical parameters were maintained and monitored daily, as follows: temperature (28℃ –28.5℃
–28.5℃ ); pH (7.0–7.5); salinity (0.25–0.75 ppt) and dissolved oxygen (4–7.8 ppm). The nitrogenous waste products were controlled through manual cleaning and changing of aquarium water once a week. The whole acclimatization period lasted for two weeks before the organism was used for experimentation. The organism was brought to the University of San Carlos Biological Museum and have been identified and certified as Danio rerio (Hamilton 1882) based on its external morphology.
); pH (7.0–7.5); salinity (0.25–0.75 ppt) and dissolved oxygen (4–7.8 ppm). The nitrogenous waste products were controlled through manual cleaning and changing of aquarium water once a week. The whole acclimatization period lasted for two weeks before the organism was used for experimentation. The organism was brought to the University of San Carlos Biological Museum and have been identified and certified as Danio rerio (Hamilton 1882) based on its external morphology.
Conditioning and acclimatization of experimental organisms
For brine shrimp, the protocol for the preparation of artificial seawater was adopted from [35] with modifications, i.e. 60 g of sea salt dissolved in two liters of distilled water. For zebrafish, the published method of [36] was adopted for the preparation of artificial water. Briefly, in a 100L distilled water, 7.5g NaHCO3, 1.8g sea salt, and 0.84g CaSO4 were dissolved and was considered a water solution used for water changing routine for the entire duration of the study. The zebrafish were acclimatized in a 24″ x 12″ x 12″ (home tank) with a division to separate each sex: male (8″ x 12″ x 12″); female (16″ x 12″ x 12″). A separate 15″ x 8″ x 12″ aquarium was used for the breeding of zebrafish. The brine shrimp used a 1334 ″ x 978
″ x 978 ″ modified tank for the hatching of its cysts.
″ modified tank for the hatching of its cysts.
Preparation of embryo medium for Zebrafish assay
For the zebrafish assay, an embryo medium was utilized for the zebrafish eggs after being taken out of the breeding tank and rinsed. Here, we adopted the preparation of Embryo Medium (E3) from [33], containing the following: 5mM sodium chloride (NaCl), 0.17 mM potassium chloride (KCl), 0.33 mM calcium chloride (CaCl2 ), 0.33 mM magnesium sulfate (MgSO4
), 0.33 mM magnesium sulfate (MgSO4 ), and 0.1% methylene blue. Upon completion of all the experiments, surviving larvae that were exposed to test solutions were euthanized by immersion in 1.2% sodium hypochlorite (bleach) to the point of loss of coordination. [33, 37]
), and 0.1% methylene blue. Upon completion of all the experiments, surviving larvae that were exposed to test solutions were euthanized by immersion in 1.2% sodium hypochlorite (bleach) to the point of loss of coordination. [33, 37]
Brine Shrimp Lethality Assay (BSLA)
For the hatchability test, the light and aerator were set to provide a good environment required for the cysts to hatch. After the hatching process, the aerator was turned off. The newly hatched nauplii were settled at the bottom of the tank and the shells float to the surface. These were siphoned and used in the experiment.
In this assay, ten nauplii were transferred in a test tube with artificial seawater (ASW) using a dropper and were exposed to different concentrations of the plant extract. After 24 hours of exposure, the number of live nauplii in each test tube was counted. For each concentration, the percent lethality of the nauplii was calculated. [35]
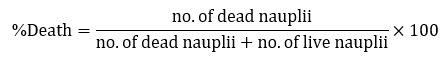
Zebrafish Assay
The care and husbandry of zebrafish were according to the published method of Brannen et al. (2013) [33]. The zebrafish were fed twice a day (6:00 a.m. and 6:00 p.m.) with fish flakes as their food. [33, 34]
The breeding and embryo collection of zebrafish was based on the published review method of Tsang et al. (2007) [38]. Briefly, the male and female (1:2) zebrafish were transferred into the breeding aquarium with a meshed rectangular box where the eggs fall through and not get eaten by the parents. The mating groups were set up late in the afternoon before the desired spawning. Most mating would take place shortly after the light comes on approximately 30 minutes. The eggs were taken out from the breeding tank one hour after spawning. It was transferred immediately in a strainer and rinsed with tap water. These eggs were moved into a petri dish and exposed in the embryo medium and were used in the experiments.
The fish embryo toxicity test (FET) was used to validate if the developmental endpoints that were chosen would be affected by C. mindanaense, I. verum, and Ocimum spp. leaf extracts. This test was a modified version of the integrated study of Hallare et al. (2005), Ceylan et al. (2014), and Basnet et al. (2017) [34, 39, 40]. The fertilized eggs were exposed to the different concentrations of the extract. The different concentrations were chosen according to the result of the range-finding test. The fertilized eggs with transparent chorion and show no sign of bubble formation were placed to their specific petri dish containing embryo medium (E3). Four hours after fertilization, blastula stage embryos were gathered and transferred to the petri dish containing the different concentrations of the extracts. Ten fertilized eggs were placed in every replicate of each treatment. The dish was covered to avoid evaporation. The morphological abnormalities were viewed at specified time points (t = 24, 48, 72, 96 hpta) of exposure after its fertilization. [34] The embryos were evaluated using a stereomicroscope and the data were recorded accordingly.
Hatchability testing was based on the published method of Venkatarajulu and Sundaram (2017) [41]. Those embryos with no heartbeat, no detachment of tail, and no somite formation were considered as dead. The hatching rate is defined as the number of embryos hatched (larvae) over the initial number of embryos multiplied by a hundred.
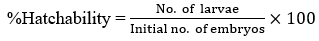
The general morphology system (GMS) scoring was used to evaluate the development of the embryo at 24, 48, 72, and 96 hpta. [42-44] The normal development of the embryo was used as the basis in scoring. [42]
For the (LC50), the indicators of zebrafish lethality and are considered as dead include; the coagulation of embryos, no heartbeat, no somite formation, and no detachment of tail. [45] This test was based on the published study of Venkatarajulu and Sundaram (2017), wherein the total numbers of dead embryos in both treatment and the controlled group were noted. [41] These data were used to find the lethal concentration (LC50) on the zebrafish embryo. The formula set by Tolentino and Undan (2016) was used to find the corrected percentage in each group. [46]
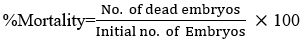
Data Analysis
For the BSLA, the lethal concentration (LC50) values were analyzed using a Probit Regression Analysis. [47] These calculations signify a preliminary result for further toxicity testing assays. [30] To evaluate the relationship between the independent variable and dependent variable from this assay, One-way ANOVA and post-hoc Tukey test was used. All this analysis was conducted using Microsoft Excel 2010 and Minitab 18.
To classify the toxicity of plant extracts, Clarkson et al. (2004) toxicity index were used. Extracts with LC50 above 1000 μ g/ml are non-toxic, LC50 of 500-1000 μ
g/ml are non-toxic, LC50 of 500-1000 μ g/ml are low toxic, LC50 of 100-500 μ
g/ml are low toxic, LC50 of 100-500 μ g/ml are medium toxic, and LC50 of 0-100 μ
g/ml are medium toxic, and LC50 of 0-100 μ g/ml are highly toxic. [48]
g/ml are highly toxic. [48]
For the Zebrafish Assay, the analysis of data was performed using Microsoft Excel 2010 and Minitab 18. All data were expressed as mean and standard deviation. One-way Univariate Analysis of Variance (One-way ANOVA) and post-hoc Turkey test were used to determine the significant differences between the treated and controlled groups. The data from the toxicity level was used to find the lethal concentration (LC50) using Probit Analysis.
Ethical Consideration
This study was reviewed and have found that the procedures were compliant to RA 8485, DA AO 40, and ACUP principles on the appropriate care and use of animals. A certificate of approval was also given by the University of San Carlos Institutional Animal Care and Use Committee (Protocol Number: 2019-020-004). Ethical clearance (CNU REC Code: 088/2018-12 Consuegra) was also given by the CNU Research Ethics Committee specified proper handling, euthanasia, and disposal of the animal models, A. salina and D. rerio.
Results and Discussion
Brine Shrimp Lethality Assay
In this study, the toxicity of C. mindanaense, I. verum, and Ocimum spp. leaf extracts were examined against the nauplii of Artemia salina. Table 1 showed that treatment 1 (1000 ppm) of CMLE (93.33%), IVLE (96.66%), and OSLE (83.33%) showed the highest mortality rate. Contrary, treatment 4 of CMLE (30%), IVLE (26.66%), and OSLE (30%) exhibited the least mortality and follows a concentration-dependent response trend. Hence, as the concentration of the leaf extract increases, the mortality rate also increases.
The LC50 value of C. mindanaense (6.961 μg /ml), I. verum (10.434 μg
/ml), I. verum (10.434 μg /ml), and Ocimum spp. (15.737 μg
/ml), and Ocimum spp. (15.737 μg /ml) signifies the lethal concentration of the leaf extract that could kill 50% of the population within 24 hours of exposure. The LC50 values were determined using probit analysis, and these values were considered to be highly toxic based on Clarkson et al. toxicity criterion. [49] Table 2 shows the mortality rate of brine shrimp (A. salina) exposed into two control groups, positive control (K2Cr2O7) displayed the highest mortality rate (100%). This control is a strong oxidizing agent and acts as a corrosive agent, which proves to be fatal. [50] Conversely, negative control (seawater) exhibits 0% mortality. Dead nauplii are determined by the absence of movement for at least 30 seconds of observation, while alive nauplii are characterized by its continuous movement and positive phototaxis. [35]
/ml) signifies the lethal concentration of the leaf extract that could kill 50% of the population within 24 hours of exposure. The LC50 values were determined using probit analysis, and these values were considered to be highly toxic based on Clarkson et al. toxicity criterion. [49] Table 2 shows the mortality rate of brine shrimp (A. salina) exposed into two control groups, positive control (K2Cr2O7) displayed the highest mortality rate (100%). This control is a strong oxidizing agent and acts as a corrosive agent, which proves to be fatal. [50] Conversely, negative control (seawater) exhibits 0% mortality. Dead nauplii are determined by the absence of movement for at least 30 seconds of observation, while alive nauplii are characterized by its continuous movement and positive phototaxis. [35]
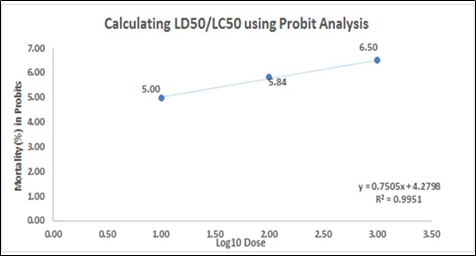
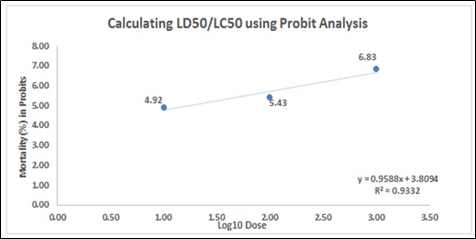
In Figure 1, the correlation coefficient (R2) of the mortality rate was plotted against the log10 of the sample concentration. The slope – (1a) y = 0.7505x + 4.2798, (1b) y = 0.9588x + 3.8094 and (1c) y = 0.6104x + 4.1608 – implies the steepness of the line and describes the linear relationship concentrations and the mortality. It also assesses the average rate of change in the dependent variable (mortality rate) as the independent variable (log concentration) changes. The y-intercept represents the mortality rate of A. salina with a value of (1a) 4.2798, (1b) 3.8094, and (1c) 4.1608. The slope value slope – (1a) 0.7505 (1b) 0.9588 and (1c) 0.6104 – depicts that as the concentration applied to the Artemia increases, the mortality rate increases by 0.7505, 0.9588 and 0.6104, respectively.
Previous studies revealed that the leaf extracts of these spices attributed its cytotoxic properties to its phytochemical compounds. Cinnamaldehyde— one of the main active compounds founds in the leaf of cinnamon increases its cytotoxic activity while coumarin, a secondary bioactive compound, has a strong carcinogenic and hepatotoxic properties. [51-53] Some of the phytochemical constituents of I. verum such as alkaloids, saponins, flavonoids, steroids, tannins proteins, and amino acids are directly responsible for its antioxidant, antiangiogenic, and anticancer properties suggesting that it exhibit cytotoxic activity. [48, 54]
Zebrafish Assay
Hatchability of Zebrafish Embryo
The percent hatchability of the embryos exposed to different concentrations of C. mindanaense, I. verum, and Ocimum spp. leaf extracts at 48-72 hpta were shown in Figure 2 where extracts at 10000 ppm showed no hatched embryos, suggesting that the concentration inhibited the normal hatching process of the embryo showing early coagulation and no heartbeat. [55] Failure of organogenesis leads to a low hatchability rate thus proving the sub-lethal property of the plant extract. [56] Morphological abnormalities could also limit the ability of the embryos to break the chorion resulting to delay or failure of hatching. [57] Furthermore, exposure of the embryos to a toxic compound found in the extract would also result in the inhibition of enzymatic activity in its hatching development. Conversely, embryos exposed to treatment 4 (10 ppm) of C. mindanaense (80%), I. verum (93.33%), and Ocimum spp. (80%) displayed the highest hatching rate. Exposure of the embryo to different concentrations of the extract affects its hatchability; as the concentration of the extract increases, the percentage hatchability decreases. Results revealed that the selected indigenous spices displayed a certain property that affects the hatching rate of the embryos.
Embryos under the control group completed their hatching process at 48 hpta. The highest percent of hatchability (96.66%) was evident in negative control since the embryo medium (E3), is the standard medium suitable for the D. rerio embryo. [33] Positive control (ethanol) exhibits a 63.33% hatching rate but early abnormalities such as pericardial edema and malformation of the tail were already evident at this period. In Table 3, all concentrations of C. mindanaense, I. verum, and Ocimum spp. leaf extract exhibited significant effects on zebrafish in terms of the hatchability rate. The results further revealed that extracts of C. mindanaense at levels of 100 ppm; I. verum at 1000 ppm and 100 ppm; Ocimum spp. at 100 ppm and 10 ppm showed the most significant activity comparable to embryo medium.
The variation of the hatchability rate in the concentrations of the extract suggests that the selected indigenous spices contain bioactive compounds. Coumarin is a bioactive component of cinnamon that has a strong carcinogenic and hepatotoxic property. [51, 53] The presence of coumarin in plant extract interrupts the hatching process of a developing zebrafish embryo. [58] Phytochemical screening also revealed the presence of flavonoids in all three selected indigenous spices.
Flavonoid is a plant component that plays a role in several biological activities. Other phytochemical constituents were also found such as tannins (C. mindanaense) and saponins (C. mindanaense and I. verum). A study conducted and revealed that the presence of phytochemical constituents such as flavonoids, tannins, and saponins affects the hatching development of Mikania glomerata. [59] Furthermore, a study by Wahyuni [60] emphasizes that the compound saponin could disrupt the growth of A. aegypti resulting in retardation or failure of hatching. Alkaloid is present in Ocimum spp., this component reveals to affect the egg hatchability of Culex pipiens, thus proving that bioactive compounds in plants contribute a sub-lethal effect on the development of an organism. [61]
Teratogenic effect of selected indigenous spices
D. rerio embryos were exposed to different concentrations and were examined after 24, 48, 72, and 96 hours of exposure. As seen in Figure 3a-3d, extracts at level 10000 ppm of CMLE, IVLE and OSLE showed early coagulation of the embryo after 24 hpta. CMLE at 1000 ppm showed pigmentation abnormality at 24 hpta and coagulation of the embryo was detected after 48 hpta. CMLE extracts at 100 ppm and 10 ppm showed normal development of the embryos although morphological abnormalities were observed such as pericardial edema and yolk sac edema at 72 hpta and 96 hpta.
In IVLE, extract with 1000ppm showed delayed growth at 48hpta; after 72 hpta and 96 hpta, morphological abnormalities were detected such as deformation of body shape and bent tail. In IVLE extracts with 100 ppm and 10 ppm, less pigmentation was observed at 24h hpta and 48 hpta; pericardial edema became evident in those treatments at 72 and 96 hpta. In the OSLE, extracts at levels 10000 ppm and 1000 ppm displayed early coagulation on most of the embryos at 24 hpta. Extracts at 100 ppm showed normal development although less pigmentation and pericardial edema were present at 72 and 96 hpta, whereas extract with 10 ppm showed deformation of body shape following rupture of zebrafish larvae at 96 hpta.
As shown in Table 4, all concentrations of C. mindanaense, I. verum, and Ocimum spp. leaf extract exhibited significant effects on zebrafish embryos in terms of teratogenicity. The different concentrations of CMLE, IVLE, and OSLE show an inversely proportional pattern where zebrafish embryos showed higher teratogenic effects at lower concentrations (100 – 10 ppm), indicating that extract at high concentration might be dangerous to the test organism.
The teratogenicity shown by the different concentrations suggests that the spices contain bioactive compounds. CMLE contains flavonoids, saponins, and tannins. According to the study conducted by [62], Cinnamomum zeylanicum has the highest phenolic and flavonoid contents compared to other herbal plants. The results of the comparative study showed a significant increase of C. zeylanicum toxic effects causing malformations on the zebrafish embryo after exposure. Another phytochemical substance found in cinnamon which is mostly responsible for its teratogenic effects is cinnamaldehyde. It has been stated in the Federal Institute for Risk Assessment [63] that high consumption of cinnamon during pregnancy could lead to any deformities of the embryos as indicated in their experiments using animals.
The phytochemicals present in the I. verum leaf extract are flavonoids, saponins, and terpenoids. I verum was said to be safe when used for pregnant and breastfeeding women as part of their diet, however, its adverse effects are often caused by overdosing its use. Also, a case report in the intoxication of I. verum on a 2-months old infant which resulted in gastrointestinal and neurological symptoms proved its toxic effects when ingested in large amounts. [64, 65] OSLE contains alkaloids, flavonoids, and terpenoids. One of these bioactive compounds may have been one of the compounds that cause teratogenicity in D. rerio in embryos. Moreover, the study of De Vera et al. (2016) stated that phytochemicals present in Ocimum sanctum (flavonoids, terpenoids, tannins, and saponins) showed teratogenic and toxic effects on D. rerio embryos. [66]
Toxic effects of the selected indigenous spices
Extracts of C. mindanaense, I. verum, and Ocimum spp. are used to test embryotoxicity. Figure 4 shows that treatment 1 (10000 ppm) of C. mindanaense, I. verum, and Ocimum spp. showed the highest mortality rate (100%). Contrary, treatment 4 (10 ppm) of C. mindanaense (6.66%), I. verum (33.33%), and Ocimum spp. (23.33%) showed the least mortality rate suggesting that the extracts exhibit a concentration-dependent response trend.
The mortality is more apparent with increasing concentration of the extracts. CMLE, IVLE, and OSLE yielded a 100% mortality at 10000 ppm after 24 hours of exposure. Embryos at 1000 ppm showed that C. mindanaense extract with consistent 50% mortality from 24 hours to 48 has 83.33% mortality at 96 hours. I. verum and Ocimum spp. extracts with 16.66% and 86.66% at 24 hours had 63% and 100% mortality at 96 hours, whereas embryos at 100 ppm showed 13.33 mortality at 24 hours in both C. mindanaense and I. verum extracts and recorded to have 20% and 33.33% mortality at the last observation period while Ocimum spp. had a consistent mortality rate of 23.33%. Embryos exposed to 10 ppm showed that C. mindanaense extract has a consistent mortality rate of 6.66% from 24 hours to 96 hours, I. verum and Ocimum spp. extracts have 6.66% and 16% at 24 hours and recorded to have 33.33% and 23.33% mortality rate at the fourth day observation period (96 hours). These data confirmed the exposure period and concentration dependency of the toxic effects of C. mindanaense, I. verum, and Ocimum spp. aqueous extract.
In table 5, the result showed that the mortality rate of D.rerio embryos and larvae between the control group and treatments of CMLE, IVLE, OSLE with different concentrations of C. mindanaense, I. verum, and Ocimum spp. aqueous extract exhibits a substantial effect on zebrafish mortality after 24, 48, 72, 96 hours of exposure, implying that the extracts were period and dose-dependent. The mortality rate of CMLE, IVLE, and OSLE vary statistically from ethanol at 10000 ppm. C. mindanaense at levels 10000 ppm and 1000 ppm, I. verum at 10000 ppm, Ocimum spp. at 10000 ppm and 1000 ppm were found to be more potent than ethanol, whereas 100 ppm and 10 ppm showed the most significant activity comparable to ethanol. From the study of Reddy (2017), most of the zebrafish embryos exposed to ethanolic solutions can survive and hatch but they develop malformation such as pericardial edema, body malformation, and other mutations. [67] The mortality between positive control and Treatment 1 differs since the extracts contain phytochemicals that are responsible for the death of zebrafish embryos and larvae.
The LC50 values of C. mindanaense (230.497 ppm), I. verum (278.328 ppm)
values of C. mindanaense (230.497 ppm), I. verum (278.328 ppm) , and Ocimum spp. (ppm) signify the lethal concentration of the leaf extracts that could kill 50% of the population within the incubation period. The LC50
, and Ocimum spp. (ppm) signify the lethal concentration of the leaf extracts that could kill 50% of the population within the incubation period. The LC50 values were determined using probit analysis, and the values were considered to be medium toxic based on Clarkson et al. toxicity criterion. [49] In Figure 5, the coefficient correlation (R2) of the mortality rate and the treatments are (5a) 0.9326 or 93.26%, (5b) 0.75 or 75% and (5c) 0 or 0%. This suggests that the zebrafish embryos/larvae mortality at 96 hours observation period is in direct relationship with concentrations of CMLE, and IVLE. However, OSLE showed no linear relationship between the concentration and the mortality but a curvilinear relationship does exist. [68] The slope – (5a) y = 1.2343x + 2.0731, (5b) y = 0.3857x + 4.055, (5c) y = 0 – illustrates the steepness of the graph and estimates the rate of change in zebrafish mortality (Y) as the concentration (X) changes. It also shows the linear relationship between concentration and mortality. The y-intercept represents the average mortality rate of D. rerio with a value of (5a) 2.0731, (5b) 4.055, and (5c) 4.2721. The slope value (5a) 1.2343, (5b) 0.3857 (5c) 0 suggests that as the concentration applied to zebrafish increases, the mortality rate will also increase with an average of 1.2343, 0.3857 and 0, respectively.
values were determined using probit analysis, and the values were considered to be medium toxic based on Clarkson et al. toxicity criterion. [49] In Figure 5, the coefficient correlation (R2) of the mortality rate and the treatments are (5a) 0.9326 or 93.26%, (5b) 0.75 or 75% and (5c) 0 or 0%. This suggests that the zebrafish embryos/larvae mortality at 96 hours observation period is in direct relationship with concentrations of CMLE, and IVLE. However, OSLE showed no linear relationship between the concentration and the mortality but a curvilinear relationship does exist. [68] The slope – (5a) y = 1.2343x + 2.0731, (5b) y = 0.3857x + 4.055, (5c) y = 0 – illustrates the steepness of the graph and estimates the rate of change in zebrafish mortality (Y) as the concentration (X) changes. It also shows the linear relationship between concentration and mortality. The y-intercept represents the average mortality rate of D. rerio with a value of (5a) 2.0731, (5b) 4.055, and (5c) 4.2721. The slope value (5a) 1.2343, (5b) 0.3857 (5c) 0 suggests that as the concentration applied to zebrafish increases, the mortality rate will also increase with an average of 1.2343, 0.3857 and 0, respectively.
The lethality shown by the different concentrations suggests that C. mindanaense, I. verum, and Ocimum spp. leaf extracts contain bioactive compounds. The major biologically active components of cinnamon are cinnamaldehyde and eugenol. [52, 53] In the study of Domaracky et al. [69], C. zeylanicum decreases the number of nuclei in female mice which is due to the antiproliferative properties of cinnamaldehyde. Laboratory animals (rats and mice) had 100% mortality within weeks of feeding cinnamaldehyde by gavage while animals that were fed with the extract orally showed lower sperm count and motility. [52] These activities are said to be dose-related. The higher dose of cinnamon extracts the higher mortality rate. Furthermore, it was shown in the study of Domaracky et al. (2007), that the mice fed with 375 mg/kg showed no sign of cytotoxic effect although there were mutations in the distribution of embryo cells. [69] Higher occurrence of dead cells was observed after the application of the clove essential oil containing eugenol as its chief bioactive component. Eugenol is considered to be the activator of apoptosis and was hypothesized to slow down activities related to the cleavage formation of the embryonic cell. [69] Another bioactive component is coumarin. High doses of coumarin intake cause liver cancer in mice and rats and lung cancer in mice. [52, 70]
Trans-anethole is the main bioactive component of I. verum. [71-73] In the study of Özguven (2012), extracts of star anise (seed) contains anethole and estragole which shows to be the cause of hepatoxicity in rodents. [74] Both phytoconstituents are structurally similar to safrole, a recognized hepatotoxic and carcinogen. Safrole can induce testicular atrophy, weight loss, depletion of bone marrow, and liver tumor in rats even at 1% concentration. [71] However, a study from Yu et al. [75] showed that essential oils of star anise – such as linalool, estragole, anethole, and limonene – are beneficial on laying performance and antioxidant status of laying hens thus prolonging the shelf life of its eggs. [76-78]
Ocimum sanctum leaf extract contains linalool, camphor, eugenol, ursolic acid, carvacrol, eugenol, estragole, and 1, 8 cineole. [66, 79] A study by [66] showed that O. sanctum possesses embryonic effects against D. rerio at a specific time and specific concentration. These embryotoxic activities could be due to its bioactive compound and essential oil such as eugenol, alkaloids, glycosides, saponin, tannins. [80] The genus of Ocimum revealed to have a significant biological property with their interesting applications as antioxidant, larvicidal, insecticidal, and anthelmintic activity. [81] A study about Ocimum sanctum leaf extract showed a positive result as an anticancer, antioxidant, and exhibited antiproliferative activity in a mouse model. [67] In the study of Mueen et al. [82], hydroalcoholic extracts of O. basilicum were screened for anti-implantation, anti-ovulatory, and anti-abortifacient activity in cyclic Wister rats. Longer diestrus phase was observed which has the potential to be developed into contraceptive and histological report showed that the rats have no anti-abortifacient and anti-implantation effect. The presence of these bioactive compounds in the extracts might have contributed to the toxicity of CMLE, IVLE, and OSLE towards the D. rerio embryo.
Conclusion:
The results of the present research provided additional evidence on the suspected toxicities of some indigenous spices especially when consumed at higher concentrations. Considering that the use of these species is widespread and popular to local communities, caution must be observed in its use—particularly if utilized in undiluted preparations. This acute toxicity investigation is an essential stage towards the limitations and use of spice in food and beverages. Further, the teratogenicity component in this study could be extended in vertebrate mammalian models to be able to infer potential ranges that could be considered safe for use. In such a manner, these indigenous spices could be fully exploited for their nutritional and nutraceutical values.
Acknowledgment:
This research would not have been possible without the generous guidance and assistance of the Cebu Normal University-Research Institute of Tropical Biology and Pharmacological Biotechnology (CNU- RITBPB) and Biology Department of the College of Arts and Sciences, Cebu Normal University. We thank the anonymous reviewers for their careful reading of our manuscript and their many insightful comments and suggestions.
References
Table 1. The number of brine shrimp nauplii that survived after treatment with Cinnamomum mindanaense, Illicium verum, and Ocimum spp. leaf extract in different concentrations and percent mortality with the median lethal concentration (LC50) value.
|
Experimental Group |
|||||||||||
|
Plant Extract |
Con cen trati on (ppm) |
Log10 Con cen trati on |
No. of Surviving nauplii after 24 hours |
Total no. Of live nau plii |
Total no. Of dead nau plii |
Total no. Of nau plii sam ples |
% Mort ality |
LC50 |
|||
|
R1 |
R2 |
R3 |
|||||||||
|
n=10 |
n=10 |
n=10 |
|||||||||
|
Cinnamomum mindanaense Leaf Extract (CMLE) |
T1 |
1000 |
3 |
0 |
1 |
1 |
2 |
28 |
30 |
93.33% |
6.961 μ
|
|
T2 |
100 |
2 |
2 |
2 |
2 |
6 |
24 |
30 |
80% |
||
|
T3 |
10 |
1 |
4 |
4 |
6 |
14 |
16 |
30 |
53.33% |
||
|
T4 |
1 |
0 |
7 |
7 |
7 |
21 |
9 |
30 |
30% |
||
|
Illicium verum Leaf Extract (IVLE) |
T1 |
1000 |
3 |
0 |
0 |
1 |
1 |
29 |
30 |
96.66% |
10.434 μ |
|
T2 |
100 |
2 |
3 |
3 |
4 |
10 |
20 |
30 |
66.67% |
||
|
T3 |
10 |
1 |
5 |
6 |
5 |
16 |
14 |
30 |
46.66% |
||
|
T4 |
1 |
0 |
7 |
8 |
7 |
22 |
8 |
30 |
26.66% |
||
|
Ocimum spp. Leaf Extract (OSLE) |
T1 |
1000 |
3 |
3 |
1 |
1 |
5 |
25 |
30 |
83.33% |
15.737 μ |
|
T2 |
100 |
2 |
3 |
2 |
5 |
10 |
20 |
30 |
66.67% |
||
|
T3 |
10 |
1 |
6 |
6 |
6 |
18 |
12 |
30 |
40% |
||
|
T4 |
1 |
0 |
7 |
6 |
8 |
21 |
9 |
30 |
30 % |
||
Legend: T = treatment; n = sample per replication; R = replicate
Table 2. The number of Brine Shrimp nauplii (A. salina) that survived after 24 hours of exposure on the different controlled groups.
|
Control Groups |
No. of surviving nauplii after 24 hours of exposure |
Total no. of alive nauplii |
Total no. of dead nauplii |
Total no. nauplii samples |
% Mortality |
|||
|
R1 |
R2 |
R3 |
||||||
|
n=10 |
n=10 |
n=10 |
||||||
|
Positive Control |
Potassium Dichromate (K2Cr2O7) |
0 |
0 |
0 |
0 |
30 |
30 |
100% |
|
Negative Control |
Seawater |
10 |
10 |
10 |
30 |
0 |
30 |
0 |
Legend: R = replicate; no. = number; n = no. of sample per replicate
1a. The percent mortality of A. salina at 24 hours, after the exposure of the different concentrations of Cinammomum mindanaense leaf extract
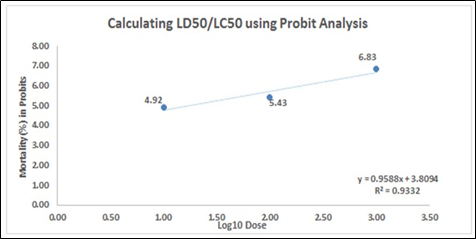
1b. The percent mortality of A. salina at 24 hours, after the exposure of the different concentrations of Illicium verum leaf extract
1c. The percent mortality of A. salina at 24 hours, after the exposure of the different concentrations of Ocimum spp. leaf extract
Figure 1. Percent mortality of A. salina at 24 hours, after the exposure of the different concentrations of (1a) Cinammomum mindanaense, (1b) Illicium verum, and (1c) Ocimum spp. leaf extracts.
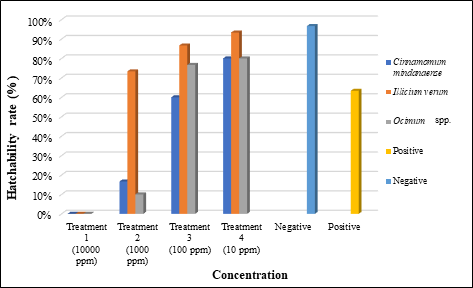
Figure 2. Percent hatchability of embryos at 48-72 hours post-treatment application in different concentrations of the selected indigenous spices leaf extract.
Table 3. Summary of the hatchability (Mean± SEM) of Danio rerio embryos at 48-72 hours post-treatment application in different concentrations of selected indigenous spices leaf extract.
SEM) of Danio rerio embryos at 48-72 hours post-treatment application in different concentrations of selected indigenous spices leaf extract.
|
GROUPS |
CONCENTRATION (ppm) |
ZEBRAFISH (Danio rerio) |
|
|
Hatchability at 48-72-hour post-treatment application |
|||
|
Cinnamomum mindanaense Leaf Extract (CMLE) |
T1 |
10000 |
0c |
|
T2 |
1000 |
1.66 ± 0.33 b, c |
|
|
T3 |
100 |
6 ± 1.73 a, b |
|
|
T4 |
10 |
8 ± 1a |
|
|
Positive Control |
|
2500 |
6.33 ± 0.88 a, b |
|
Negative Control |
|
… |
9.66 ± 0.33a |
|
ANOVA (p≤0.05) |
|
… |
0.000* |
|
Illicium verum Leaf Extract (IVLE) |
T1 |
10000 |
0c |
|
T2 |
1000 |
7.33 ± 0.66 a, b |
|
|
T3 |
100 |
8.66 ± 0.33 a, b |
|
|
T4 |
10 |
9.33 ± 0.66 a |
|
|
Positive Control |
|
2500 |
6.33 ± 0.88 a, b |
|
Negative Control |
|
… |
9.66 ± 0.33a |
|
ANOVA (p≤0.05) |
|
… |
0.000* |
|
Ocimum spp. Leaf Extract (OSLE) |
T1 |
10000 |
0c |
|
T2 |
1000 |
1c |
|
|
T3 |
100 |
7.66 ± 0.33 a, b |
|
|
T4 |
10 |
8 ± 0.57 a, b |
|
|
Positive Control |
|
2500 |
6.33 ± 0.88 a, b |
|
Negative Control |
|
… |
9.66 ± 0.33a |
|
ANOVA (p≤0.05) |
|
… |
0.000* |
Using ANOVA at (p≤ 0.05), * indicates a significant difference between control and treatment groups. Means±
0.05), * indicates a significant difference between control and treatment groups. Means± SEM with different letter superscript (a, b, c) in each spice within groups indicate a significant difference at p≤
SEM with different letter superscript (a, b, c) in each spice within groups indicate a significant difference at p≤ 0.05 using the PostHoc Tukey HSD Test.
0.05 using the PostHoc Tukey HSD Test.
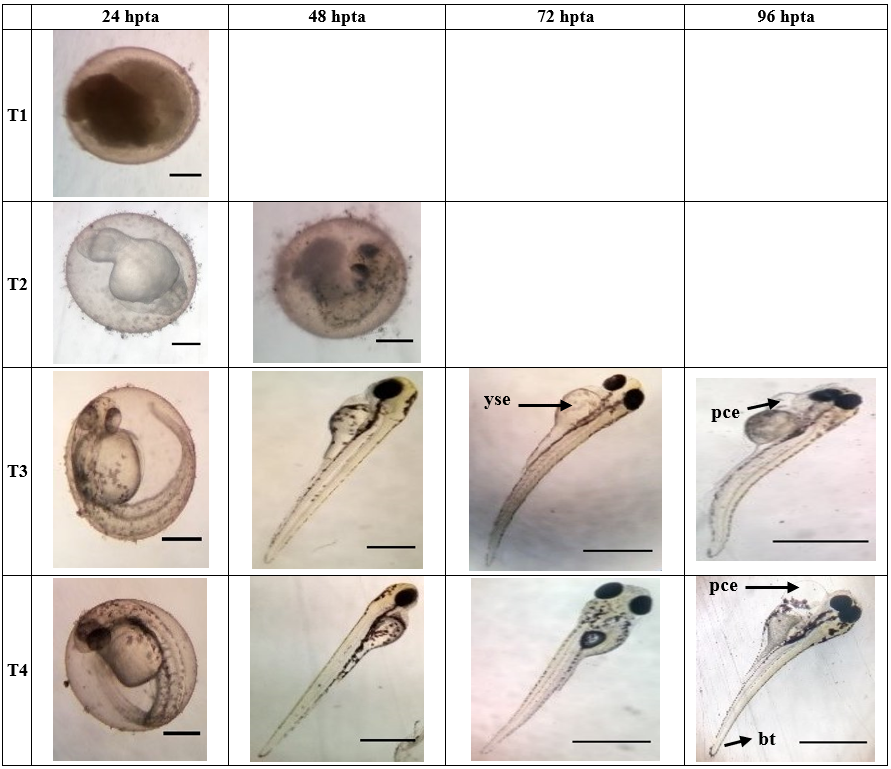
Figure 3a. Morphological development of zebrafish embryo affected by the different concentrations (T1;10 000 ppm, T2;1 000 ppm, T3;100 ppm, T4;10 ppm) of Cinnamomum mindanaense after 24, 48, 72, 96 hours post treatment application. Arrowhead points to organ with teratogenicity, such as; (yse) yolk sac edema, (pce) pericardial edema, and (bt) bent tail malformation. T1 (24hpta), T2 (24 & 48 hpta), T3 (24 hpta), T4 (24 hpta): Scale Bar = 0.7 mm. T3 (48-96 hpta), T4 (48-96 hpta): Scale Bar = ~3 mm.
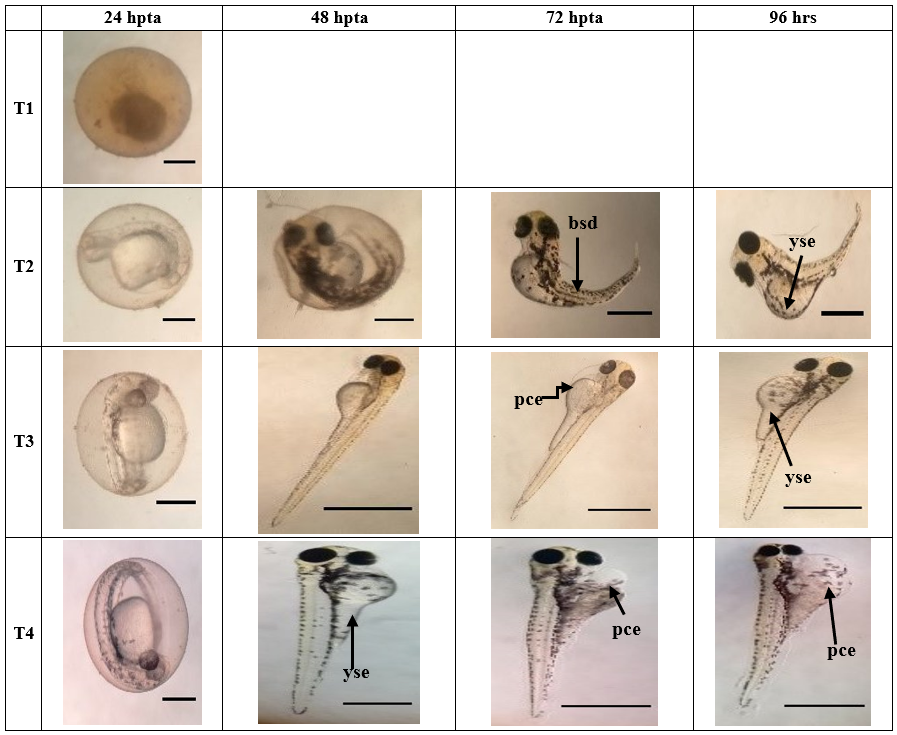
Figure 3b. Morphological development of zebrafish embryo affected by the different concentrations (T1;10 000 ppm, T2;1 000 ppm, T3;100 ppm, T4;10 ppm) of Illicium verum after 24, 48, 72, 96 hours post treatment application. Arrowhead points to organ with teratogenicity, such as; (bsd) body shape deformation, (yse) yolk sac edema, and (pce) pericardial edema. T1 (24hpta), T2 (24 & 48 hpta), T3 (24 hpta), T4 (24 hpta): Scale Bar = 0.7 mm. T2 (72-96 hpta), T3 (48-96 hpta), T4 (48-96 hpta): Scale Bar = ~3 mm.
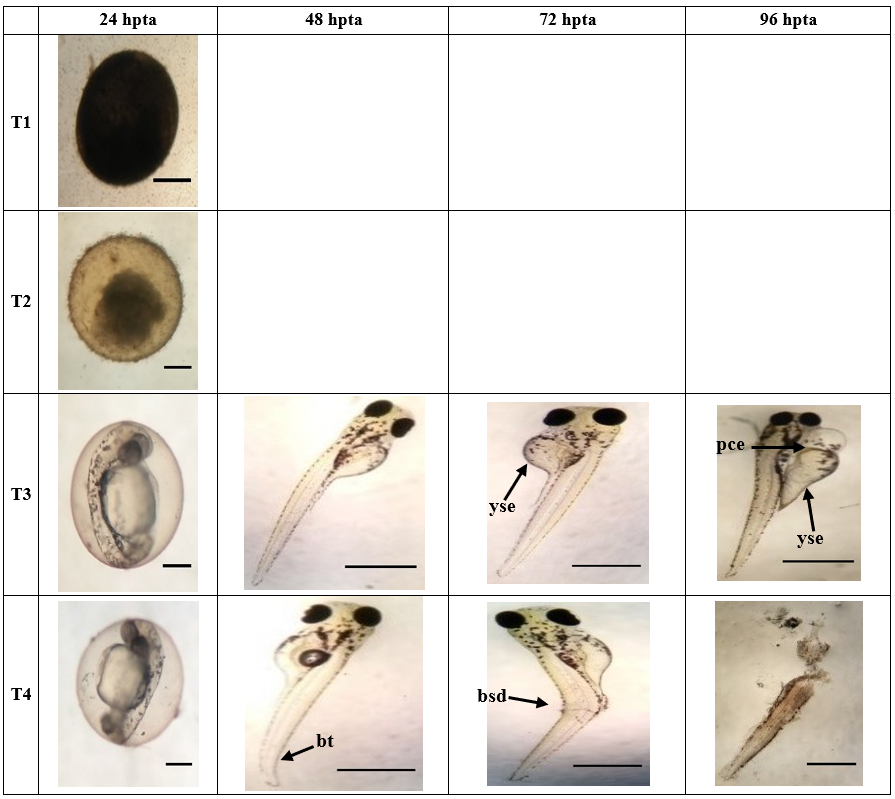
Figure 3c. Morphological development of zebrafish embryo affected by the different concentrations (T1;10 000 ppm, T2;1 000 ppm, T3;100 ppm, T4;10 ppm) of Ocimum spp. after 24, 48, 72, 96 hours post treatment application. Arrowhead points to organ with teratogenicity, such as; (yse) yolk sac edema, (pce) pericardial edema, (bsd) body shape deformation and ruptured zebrafish larvae. T1 (24hpta), T2 (24 & 48 hpta), T3 (24 hpta), T4 (24 hpta): Scale Bar = 0.7 mm. T3 (48-96 hpta), T4 (48-96 hpta): Scale Bar = ~3 mm.
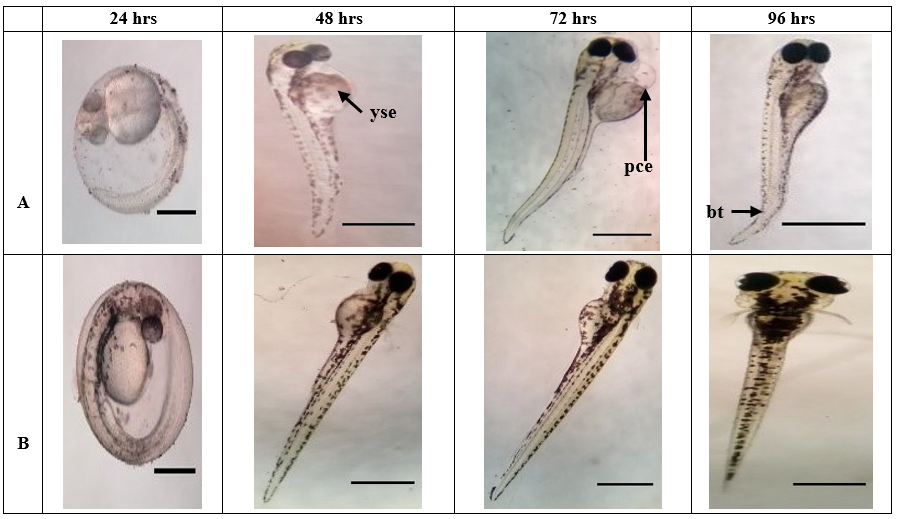
Figure 3d. Morphological malformations of zebrafish embryo as overserved in (a) positive control, such as (yse), yolk sac edema, (bt) bent tail, and (pce) pericardial edema. Whereas, figure – (b) negative control, showed normal embryonic development of zebrafish. Positive & Negative Control (24 hpta): Scale Bar = 0.7 mm. Positive & Negative Control (48-96 hpta): Scale Bar = ~3 mm.
Table 4. Summary of Danio rerio teratogenicity of the varying concentrations of Cinnamomum mindanaense, Illicium verum, and Ocimum spp. leaf extracts and control groups.
|
GROUPS |
CONCENTRATION (ppm) |
ZEBRAFISH (Danio rerio) |
|
|
Teratogenicity after 96 hours of exposure (mean±SEM) |
|||
|
Cinnamomum mindanaense Leaf Extract |
10000 |
0±0c |
|
|
1000 |
13.75±5.41b, c |
||
|
100 |
50.17±12.07a, b |
||
|
10 |
64.92±14.25 a |
||
|
Positive Control |
2500 |
43.83±10.11a, b, c |
|
|
Negative Control |
… |
75.75±15.86a |
|
|
ANOVA (p≤0.05) |
… |
0.000* |
|
|
Illicium verum Leaf Extract |
10000 |
0±0b |
|
|
1000 |
34.75±9.21a, b |
||
|
100 |
43.25±12.31a, b |
||
|
10 |
56.83±15.14 a |
||
|
Positive Control |
2500 |
43.83±10.11a, b |
|
|
Negative Control |
… |
75.75±15.86a |
|
|
ANOVA (p≤0.05) |
… |
0.001* |
|
|
Ocimum spp. Leaf Extract |
10000 |
0±0b |
|
|
1000 |
0±0b |
||
|
100 |
49.67±11.07a |
||
|
10 |
53.42±11.33 a |
||
|
Positive Control |
2500 |
43.83±10.11a |
|
|
Negative Control |
… |
75.75±15.86a |
|
|
ANOVA (p≤0.05) |
… |
0.000* |
Using ANOVA at (p≤ 0.05), * indicates a significant difference between control and treatment groups. Means±
0.05), * indicates a significant difference between control and treatment groups. Means± SEM with different letter superscript (a, b, c) in each spice within groups indicate a significant difference at p≤
SEM with different letter superscript (a, b, c) in each spice within groups indicate a significant difference at p≤ 0.05 using the PostHoc Tukey HSD Test.
0.05 using the PostHoc Tukey HSD Test.
|
4a. Percent mortality of Danio rerio at 24, 48, 72 and 96 hours after the exposure of the different concentrations of Cinammomum mindanaense leaf extract. |
|
4b. Percent mortality of Danio rerio at 24, 48, 72 and 96 hours, after the exposure of the different concentrations of Illicium verum leaf extract. |
|
4c. Percent mortality of Danio rerio at 24, 48, 72 and 96 hours, after the exposure of the different concentrations of Ocimum spp. leaf extract. |
Figure 4. Percent mortality of Danio rerio at 24, 48, 72, and 96 hours, after the exposure of the different concentrations of (4a) Cinammomum mindanaense, (4b) Illicium verum, and (4c) Ocimum spp. leaf extracts.
Table 5. Summary of Danio rerio embryo and larvae mortality of the varying concentrations of Cinnamomum mindanaense, Illicium verum, and Ocimum spp. leaf extract and control groups.
|
GROUPS |
CONCENTRATION (ppm) |
ZEBRAFISH (Danio rerio) |
||||
|
Mortality after hours of exposure |
||||||
|
24 |
48 |
72 |
96 |
|||
|
Aqueous Extracts of Indigenous Spices |
10000 |
C. mindanaense |
10±0a |
10±0a |
10±0a |
10±0a |
|
I. verum |
10±0a |
10±0a |
10±0a |
10±0a |
||
|
Ocimum spp. |
10±0a |
10±0a |
10±0a |
10±0a |
||
|
1000 |
C. mindanaense |
5±0.58b |
5±0.58b |
8.33±0.33a |
8.33±0.33a |
|
|
I. verum |
1.67±0.33b |
2.67±0.67b, c |
4±1.15b |
6.33±0.33b |
||
|
Ocimum spp. |
8.67±0.33a |
9±0a |
9.33±0.33a |
10±0a |
||
|
100 |
C. mindanaense |
1.33±0.88c |
1.33±0.88c, d |
2±0.58b, c |
2±0.58b, c |
|
|
I. verum |
1.33±0.33b |
1.33±0.33b, c |
2.33±0.33b, c |
3.33±0.33b, c |
||
|
Ocimum spp. |
2.33±0.33b, c |
2.33±0.33b, c |
2.33±0.33b, c |
2.33±0.33b, c |
||
|
10 |
C. mindanaense |
0.67±0.67c |
0.67±0.67c, d |
0.67±0.67c |
0.67±0.67c |
|
|
I. verum |
0.67±0.67b |
0.67±0.67c |
1.33±0.88b, c |
3.33±1.45b, c |
||
|
Ocimum spp. |
1.67±0.33b, c |
2±0.58b, c |
2±0.58b, c |
2.33±0.33b, c |
||
|
Positive Control (Ethanol) |
2500 |
C. mindanaense |
3±1.15b, c |
3.67±0.88c |
3.67±0.88b |
4±1b |
|
I. verum |
3±1.15b |
3.67±0.88b |
3.67±0.88b |
4±1b, c |
||
|
Ocimum spp. |
3±1.15b |
3.67±0.88b |
3.67±0.88b |
4±1b |
||
|
Negative Control (Embryo Medium) |
… |
C. mindanaense |
0.33±0.33c |
0.33±0.33d |
0.33±0.33c |
0.67±0.33c |
|
I. verum |
0.33±0.33b |
0.33±0.33c |
0.33±0.33c |
0.67±0.33c |
||
|
Ocimum spp. |
0.33±0.33c |
0.33±0.33c |
0.33±0.33c |
0.67±0.33c |
||
|
ANOVA |
C. mindanaense |
0.000* |
0.000* |
0.000* |
0.000* |
|
|
I. verum |
0.000* |
0.000* |
0.000* |
0.000* |
||
|
(p≤0.05) |
Ocimum spp. |
0.000* |
0.000* |
0.000* |
0.000* |
|
|
LC50 |
C. mindanaense |
230.497 ppm |
||||
|
I. verum |
278.328 ppm |
|||||
|
Ocimum spp. |
… |
|||||
ANOVA at (p≤0.05), * indicates a significant difference between the control group and the treatment groups. Using PostHoc analysis, different letter superscripts (a, b, c, d) indicates a significant difference at p≤0.05. Means that do not share the same letter are significantly different.
|
5a Percent mortality of Danio rerio at 96 hours, after the exposure of the different concentrations of Cinammomum mindanaense leaf extract |
|
5b Percent mortality of Danio rerio at 96 hours, after the exposure of the different concentrations of Illicium verum leaf extract |
|
5c Percent mortality of Danio rerio at 96 hours, after the exposure of the different concentrations of Ocimum spp. leaf extract
|
Figure 5. Percent mortality of Danio rerio at 96 hours, after the exposure of the different concentrations of (5a) Cinammomum mindanaense, (5b) Illicium verum, and (5c) Ocimum spp. leaf extracts
