The Diet-Induced Gut Microbiota Diversity Improved Glycemic Control: A Meta-Analysis
Hyder Osman Mirghani 1, Saif Atallah S Alatawi 2*, Khalil Fathi Alsharef 2
|
|
|
ABSTRACT
There is an increasing awareness of personalized nutrition on glycemic control. This review aimed to assess the effect of diet on gut microbiota and glycemic control among patients with type 2 diabetes. PubMed, Medline, and Google Scholar were searched for relevant articles; the keywords gut microbiota, gut microbiome, prebiotics, probiotics, fiber diet, fasting plasma glucose (FPG), postprandial plasma glucose, the glycated hemoglobin, and HbA1c were used. From among the retrieved studies, 40 full texts were screened and only 17 fulfilled the inclusion and exclusion criteria, and the extraction sheet was used to report the author's names, year of publication, country, number of patients, and the duration of the study. The meta-analysis showed the significant effect of probiotics on reducing HOMA-IR, standardized mean difference (SMD), –0.35; CI –0.68 to –0.02, P=0.04; I 2 = 21%; P=0.28 for heterogeneity, and fasting insulin, (SMD), -8.0; CI, -10.05 to- 5.96; P <0.00001. The included studies showed 82.0% heterogeneity, P=(0.0002), FPG, (SMD), -2.78; confidence interval (CI), -9.49 to 3.92, P=0.42; I 2 = 77%; P=0.0002 for heterogeneity), and gut microbiota, (SMD), 0.49; confidence interval (CI), 0.27 to 0.91, P = 0.02; I 2 = 0.0%; P=1.00 for heterogeneity). However, no effects were observed regarding HbA1c levels, (SMD), 0.50; confidence interval (CI), –16 to 1.16, P = 0.14; I 2 = 89%; P <0.0001 (for heterogeneity), Lactobacillales and Bifidobacterium were associated with good outcomes, while Firmicutes were linked to poor glycemic control. Conclusion: Diet positively affected microbiota diversity, FPG, and insulin resistance; no effects were evident regarding HbA1c.
Keywords: Fiber diet, Probiotics, Fermented milk, Type 2 diabetes, Microbiota diversity, Glycemic control
Introduction
Diabetes mellitus is becoming an epidemic; it affects 285 million people worldwide and expected to jump to 438 million by the year 2030 [1-4]. There are billions (two kilograms) of microbiome in the human body (microbiota). Microbiota is considered as an endocrine organ regulating the function of the human body; it is linked to various diseases including type 2 diabetes mellitus (T2DM), Alteration of the microbiota composition changes the host response to lipid, and carbohydrate metabolism [5, 6].
Randomized controlled trials showed that serum indole propionic acid (IPA), a microbial metabolite of tryptophan, was linked to a lower likelihood of developing T2DM probably mediated by a direct effect on beta-cell function or the interplay between dietary fiber and inflammation [7]. In addition, exercise was found to control blood sugar by modifying the gut microbiota composition, low-grade inflammation and endotoxemia reduction, and decreased intestinal permeability [8, 9]. Moreover, metformin the first-line antidiabetic medication was shown to exert some of its effects through microbiota alteration among naïve T2DM [10]. The relative abundances of Lactobacillus and Bifidobacterium in the gut microbiota and depletion of Bacteroides lead to gene alteration in bile acid metabolism and increased insulin sensitivity [11]. Further studies showed that weight loss among patients with T2DM significantly reduced Collinsella species that increase the risk of atherosclerosis [12]. A duodenal-jejunal bypass surgery with minimal gastric resection increased A. muciniphila of gut microbiota and positively affects cardio-metabolic risk factors [13]. Additionally, bariatric surgery was shown to exert more effects on microbiota diversity among patient with diabetes and a high body mass index [14] compared to medical treatment, besides, increasing butyrate-producing microbes by probiotics was shown to play a major role in diabetes control [15].
Few reviews assessed the relationship between diet, gut microbiota and glycemic control. Zuhang et al. [16] assessed the relationship between choline metabolite trimethylamine N-oxide produced by microbiota and glycemic control. Houghton et al. [17] focused on dietary interventions and exercise on glycemic control and included RCTs and other studies. Two reviews assessed the effects of probiotics on glycemic control and included experimental and animal studies [18, 19]. Three reviews assessed the effects of bariatric surgery on microbiota diversity [20-22]. A review investigated the effects of grain fiber on microbiota composition [23]. A recent study assessed the effects of microbiota on metabolic syndrome [24]. Considering the above and the notion that universal dietary recommendations may have limited utility and the suggestion for personalized nutrition to control postprandial blood glucose, we conducted this review to assess the effects of diet on the gut microbiota diversity and glycemic control among patients with type 2 diabetes.
Methodology:
The review was reported using Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) [25]. Search strategy: a qualitative systemic review was conducted in the PubMed, Medline, and Google Scholar databases for randomized controlled studies published in the English language and conducted among adults with type 2 diabetes mellitus. The search was limited to studies published during the period from January 2010 up to September 2020. The keywords gut microbiota, gut flora, type 2 diabetes mellitus, glycemic control, fasting plasma glucose, postprandial plasma glucose, glycated hemoglobin, diet, probiotics, gut microbiota modulation, and gut microbiota diversity connected via the logical (Boolean) operator “AND” or “OR”. The references and citations were also searched manually and articles fulfilling the inclusion criteria were included.
Data handling, Selection Criteria, and Analysis:
Articles were selected based on the following inclusion criteria: Intervention studies with probiotics compared to placebo with an RCT design, interventional studies among adults with type 2 diabetes, the studies were excluded if they were conducted among children or pregnant women, on the metabolic syndrome, non RCTs, or without a placebo arm. No limitations were applied to the type of diet used (prebiotics, probiotics, fiber diets, fermented milk, Chinese remedies were also included). The measurement of the gut microbiota in stool was a requirement; however, we did not specify any criteria for the diagnosis of type 2 diabetes due to the various methods for the diagnosis. The patients should be diagnosed with diabetes either naïve or on medications, controlled or not.
The primary outcomes were:
Effects on FPG, HbA1c, insulin resistance (HOMA-IR), and gut microbiota diversity
Data Extraction: Details of the author name, country, year of publication, study population, the study duration, interventions, comparators, and outcomes were captured using a data-extraction form. The data detaining the number of subjects (control versus test group), and values of tested of each parameter before and after the administration of a diet that is thought to influence the microbiota diversity.
Quality and Risk of Bias Assessment: Jadad criteria for randomized controlled trials were used, the criteria were five (yes/no), and each positive answer is given one point with a maximum of five points (indicating good quality). The items are, randomization, double-blinding, the description of double-blinding and randomization, and description of withdrawal and dropout, when the methods of randomization and blinding are not appropriate, one point is to be deducted for each [26].
Statistical Analyses: RevMan 54 software was used for the meta-analysis. For microbiota diversity (binary) risk ratios (RRs) with 95% confidence intervals (CIs) were combined across relevant studies; the continuous studies (FPG, HbA1c, and HOMA-IR, the differences in pre-post changes between the probiotics and placebo groups were combined; the fixed effects module was applied unless if substantial heterogeneity was found (A P value ≤ 0.10 for Cochran’s Q test or an I2 ≥ 50% was suggestive). A two-tailed P < 0 05 was considered statistically significant for all analyses except heterogeneity tests. The different phases of the review process were shown in figure 1.
Results:
From among the 788 articles retrieved, 40 full texts were assessed, and 17 fulfilled the inclusion and exclusion criteria; nearly two-thirds (62.5%) were from Asia, 25% from Europe and the rest were published in the USA and South America. The studies included 1395 patients with type 2 diabetes mellitus with a mean duration of 13.11±11.10 months (range 4-50), thirteen studies (81.3%) affected the microbiota diversity, 6 studies used probiotics, three administered Chinese herbal remedies, two used fermented milk, one tested the effects of sardines, another one tested acarbose the antidiabetic medication, one study tested a low branched=chain amino acid, and two studies used a high fiber diet. Eleven studies showed an improvement in either FPS or glycated hemoglobin, and one study improved insulin sensitivity (75%) and 25% showed no improvement. All the studies reported an improvement in insulin sensitivity. The microbiota that increased were Lactobacillales, Bifidobacterium, Faecalibacterium prausnitzii, Enterococcus faecalis, Bacteroidetes, E.Coli, Shirota, and Clostridium, the strains decreased were phylum Firmicutes, the Clostridium cluster IV and subclusterXIVa, Alistipes, Parabacteroides, Pseudobutyrivibrio, and Bacteroidetes. It is interesting to note that the abundance of Firmicutes is associated with no reduction in glycemic measures (Table 1 & 2).
Gut microbiota effects on fasting blood glucose: Out of the eight studies included (362 participants), six studies showed improvement [27-32], while two showed no effect [33, 34]. A fixed effect meta-analysis showed no effect of diet on FPG, (SMD, -2.78; CI, -9.47 to -3.92; P = 0.42; the included studies showed 0.0% heterogeneity, P=1.0 (Figure 2).
Gut microbiota effects on the glycated hemoglobin (HbA1c): Out of the eight studies included (342 participants), five studies showed improvement [29-33], while one showed no effect [34]. A fixed-effect meta-analysis showed that the diet improved the glycated hemoglobin, (SMD, 0.16; CI, 0.00 to 0.33; P = 0.05; the included studies showed 69.0% heterogeneity, P=0.006 (Figure 3).
Microbiota diversity (induced by diet) effects on insulin resistance as measured by (HOMA-IR): Out of the six studies included, five studies showed a reduced insulin resistance [29, 31, 32, 34, 35], and one study showed no effect [36]. The overall effect was positive ((SMD, -0.35; CI, -0.68 to 0.02; P = 0.04; the included studies showed 21.0% heterogeneity, P=0.28) (Figure 4).
Figure 5 depicted the effects of gut microbiota on fasting insulin level, four studies showed a reduction of insulin level, and one showed an increasing level. The total number of patients was (304), the overall effect was positive ((SMD, -8.0; CI, -10.05 to- 5.96; P <0.00001; the included studies showed 82.0% heterogeneity, P=0.0002).
Discussion:
The current meta-analysis showed that the diet affected the gut microbiota diversity resulting in improvement of the glycated hemoglobin, insulin resistance, and fasting insulin levels; however, no improvement was observed regarding the fasting plasma glucose. Furthermore, significant heterogeneity was observed among the studies assessing the FPG, HbA1c, and insulin levels.
The Effects of Gut Microbiota Disruption on Insulin Resistance and Fasting Insulin Levels:
Our study found a beneficial effect of diet-induced gut microbiota disruption of insulin resistance, five studies showed a reduction in insulin resistance (27, 28, 29, 34, & 36), and one study showed no effect (35). The overall effect was positive ((SMD, -0.35; CI, -0.68 to 0.02; P = 0.04; the included studies showed 21.0% heterogeneity, P=0.28, the present findings are in line with previous studies [37-39], an improvement in fasting insulin levels was also observed in the current data, four studies showed a reduction of insulin level, and one showed an increasing level. The total number of patients was (304), the overall effect was positive ((SMD, -8.0; CI, -10.05 to- 5.96; P <0.00001; the included studies showed 82.0% heterogeneity, P=0.0002 in line with Tabrizi and colleagues findings, and Zhang et al. [38, 40].
The Effects of Gut Microbiota Disruption on Glycemic Control: It is understood that the diet-induced microbiota effects start as earlier as 24 hours, the mechanisms of action might be through branched-chain amino acids, anti-inflammatory, and immune modulation [41-44]
The current findings showed conflicting results regarding glycemic control. A significant net effect was observed on the HbA1c; however, no effects were shown on fasting plasma glucose, SMD, 0.16; CI, 0.00 to 0.33; P = 0.05. The included studies showed 69.0% heterogeneity, P=0.006, and SMD, -2.78; CI, -9.47 to -3.92; P = 0.42. The included studies showed 0.0% heterogeneity, P=1.0 respectively, the current findings are in agreement with Zhang et al. [38] and Kasinska et al. [39], and in contradiction to Akbari et al. [45] who found an improvement in both FPG and HbA1c, and Ardeshirlarijani et al. [46] who observed a modest improvement on FPG and non-significant effects on HbA1c. The discrepancy regarding the positive effects on the glycated hemoglobin and the FPG may be explained by the fact that the glycated hemoglobin reflects the glycemic status over 2-3 months, while FPG only reflects a shorter period. Studies using continuous plasma glucose monitoring reflecting the true time in the range may resolve the issue.
The Microbiota Profile among Patients and Control before and after the Diet (Probiotics Consumption):
Previous studies reported that the diet substantially affected gut microbiota diversity in as early as 24 hours. Besides, these effects reverse to normal in 48 hours pointing to a rapid effect, the mechanism of gut microbiota on metabolic parameters may be mediated by reducing the body weight; other animal studies found a reduction in plasma levels of lipopolysaccharide-binding protein, a marker of endotoxemia, a reduced intestinal inflammatory activity index was reported by other animal studies [47-49].
Sasaki et al. [33] observed the abundance of Lactobacillales and Bifidobacterium species and the reduction of Clostridium clusters among patients with diabetes with no significant difference from the placebo group. Also no difference between the placebo and the diet group (Aspergillus niger transglucosidase) indicating a constant lineage of bacteria within each individual. However, the Bacteroidetes-to-Firmicutes ratio significantly increased among patients compared to controls. Zhao et al. observed the abundance of short-chain fatty acid-producing microbiota after a high fiber diet [36]. Balfego and colleagues [29] showed no difference in the abundance of gut microbiota following a sardine diet with significant reduction Firmicutes in both groups with a reduction in Firmicutes/Bacteroidetes. Clostridium and Lactobacillus abundance in the feces and reduction in the blood after fermented milk consumption were found in a study conducted in Japan [30] that indicated the reduction of bacterial translocation. Other mechanisms of fermented milk regarding its effects on diabetes might be through inflammatory cytokines (TNF-α & resistine) and increase in the acetic acid [50].
A study conducted in Sweden [51] showed no change in microbiota diversity or overall microbiota composition after L. reuteri treatment; however, secondary bile acid deoxycholic acid was increased. A galactooligosaccharides mixture did not affect microbiota abundance in a previous study in which Veillonellaceae was correlated with plasma glucose reduction. The authors attributed their results discrepancy compared to previous literature to background diet, the dose, and duration of the prebiotic, and the methods of microbiota analysis [34], another method for improving plasma glucose is the increments of serum sirtuin1 and fetuin-A induced by a diet containing Lactobacillus casei [32]. Xu et al. used a Chinese herbal remedy and found increasing Faecalibacterium spp [28]. Xu and colleagues' observation was supported by Tong et al. [31] using another herbal remedy. It is interesting to note that acarbose the antidiabetic medication was shown to increase Bifidobacterium longum and Enterococcus faecalis [52]. The current data were similar to the meta-analysis conducted by Singh et al. [53].
The strength of the current study is that the studies were randomized and double-blinded with high quality (Jadad, 4-5), and most of the doses of the diet were clearly stated and different volumes (low-dose, medium, & high), and the follow-up during the study was clear and appropriate.
The Study Limitations: The study was limited by the diversity of the patient's background (drug-naïve, on medications, & different body mass index), and the different methods of the included studies.
Conclusion:
Evidence from randomized controlled trials showed that the diet composition (by high fiber, probiotics, & fermented milk) greatly improved the gut microbiota diversity, improved insulin resistance, and glucose tolerance among patients with T2DM. The microbiota that increased were Lactobacillales, Bifidobacterium, Faecalibacterium prausnitzii. The decreased strains were phylum Firmicutes, the Clostridium cluster IV and subcluster XIVa, Alistipes, Parabacteroides, Pseudobutyrivibrio, and Bacteroidetes. It is interesting to note that the abundance of Firmicutes is associated with no reduction in glycemic measures. The patients and microbiota characteristics at the baseline, the gut permeability, and the state of bacteremia may greatly influence the results. Further, larger studies controlling various confounders and using continuous glucose monitoring are needed.
Key Messages (Provide appropriate messages of about 35-50 words to be printed in centre box):
Microbiota disruption by probiotics, a high fibrer diet, and some herbal remedies might be a cheap, safe, and efficient intervention (personalized nutrition) for glycemic control among patients with type 2 diabetes. In addition, it can be introduced in prediabetes stage to increase insulin sensitivity.
Conflicts of Interest: Nil
Acknowledgement:
The authors would like to acknowledge the Saudi digital library for accessing the data included in this manuscript.
References
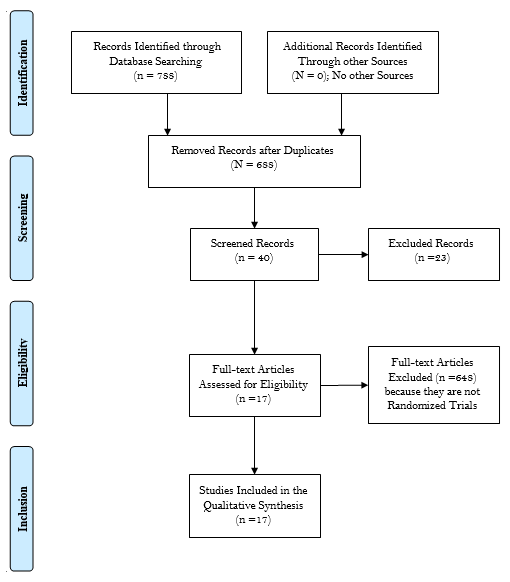
Figure 1 - Flow Diagram through the Different Phases of the Systematic Review (PRISMA Flowchart).
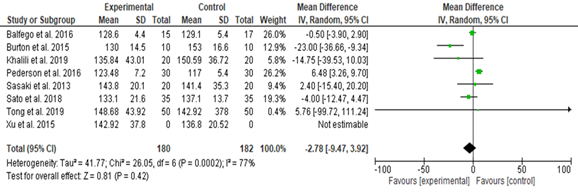
Figure 2. Effects of Diet on the Fasting Plasma Glucose among the Study Group (Random Effect)
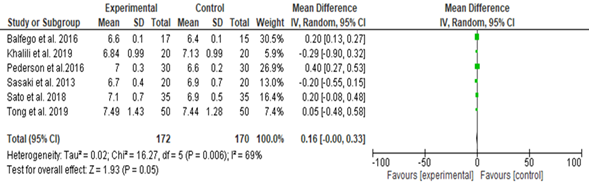
Figure 3. Effects of Diet on the Glycated Hemoglobin among the Study Group
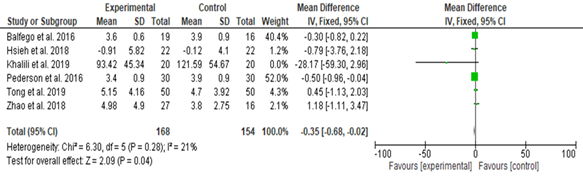
Figure 4. The Effects of Diet on Insulin Resistance
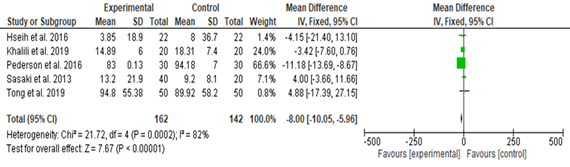
Figure 5. The Effects of Gut Microbiota Disruption on Fasting Insulin Levels
Table 1. The Probiotics Effects on the Gut Microbiota among Patients with Type 2
|
Author |
Year |
Country |
Sample |
duration |
Patients |
The quality and risk of bias (Jadad) |
|
Sasaki et al. |
2013 |
Japan |
60 |
12 |
T2DM on drugs |
5 |
|
Burton et al. |
2015 |
USA |
|
4 |
T2DM on drugs or lifestyles |
4 |
|
Xu et al. |
2015 |
China |
187 |
12 |
T2DM drug-naive |
5 |
|
Su et al. |
2015 |
China |
95 |
4 |
T2DM on treatment |
4 |
|
Hsieh et al. |
2016 |
Taiwan |
68 |
12 |
T2DM no treatment |
5 |
|
Balfego et al. |
2016 |
Spain |
35 |
24 |
T2DM not on treatment |
4 |
|
Pedersen et al. |
2016 |
UK |
29 |
12 |
T2DM on treatment |
5 |
|
Firouzi et al. |
2017 |
Malaysia |
136 |
12 |
T2DM on treatment |
5 |
|
Mobini et al. |
2017 |
Sweden |
46 |
12 |
T2DM on insulin |
5 |
|
Tonucci et al. |
2017 |
Brazil |
50 |
6 |
T2DM |
5 |
|
Sato et al. |
2017 |
Japan |
70 |
16 |
T2DM |
4 |
|
Tong et al. |
2018 |
China |
450 |
12 |
T2DM |
4 |
|
Zhou et al. |
2018 |
China |
43 |
8 |
T2DM |
4 |
|
Razmpoosh et al. |
2019 |
Iran |
60 |
6 |
T2DM |
5 |
|
Karusheva et al. |
2019 |
Germany |
12 |
4 |
T2DM |
5 |
|
Khalili et al. |
2019 |
Iran |
40 |
8 |
T2DM |
5 |
|
Shin et al. |
2020 |
Korea |
|
20 |
T2DM on metformin |
5 |
Table 2. The Mode of Therapy, the Effect on Gut Microbiota, and Effects on Plasma Sugar and Insulin Sensitivity in the Review
|
Diet used |
Bacteria increased |
Bacteria decreases |
Effect on DM |
|
Aspergillus niger transglucosidase to form undigested fiber in the GI tract (23) |
Lactobacillales and Bifidobacterium |
The Clostridium cluster IV and subclusterXIVa |
A non-significant reduction in the HbA1c (P=0.07) |
|
Inulin from agave, beta-glucan from oats, and polyphenols from blueberry pomace (24) |
- |
- |
Fasting plasma sugar improved (P=0.02), increased metformin tolerance |
|
Gegen Qinlian Decoction (Chinese herbal formula) (25) |
Faecalibacterium prausnitzii, |
Alistipes, Parabacteroides, and Pseudobutyrivibrio |
FPS and HbA1c significantly decreased (P<0.01, and 0.05 respectively, PPPs not affected) |
|
Acarbose (26) |
Bifidobacterium longum and Enterococcus faecalis |
- |
Decrease some inflammatory cytokine independent of antihyperglycemic effects. |
|
Lactobacillus reuteri ADR-3 and ADR-1(27) |
Bifidobacterium spp. and Lactobacillus spp |
Bacteroidetes |
HbA1c reduced with ADR-1 (P=0.021), no significant reduction in FPS |
|
Sardine enriched diet (28) |
Bacteroidetes, and E.Coli |
phylum Firmicutes |
No improvement in HbA1c and FPS (P=0.0.08 and 0.18 respectively) |
|
Galactooligosaccharides mixture (29) |
Veillonellaceae high but not significant |
No significant change |
No significant changes in FPS (P=0.221) |
|
Multi-strain probiotics (30) |
The Lactobacillus and Bifidobacterium |
- |
HbA1c significantly improved (P<0.05) |
|
Lactobacillus reuteri DSM 17938 (31) |
No effect |
No effect |
No change in HbA1c |
|
Fermented milk (32) |
Lactobacillus acidophilus La-5 and Bifidobacterium animalis subsp lactis BB-12 |
- |
Fructosamine and HbA1c levels decreased (P=0.04 and 0.02 respectively) |
|
Fermented milk (33) |
Lactobacillus casei strain Shirota, Clostridium coccoides, and Clostridium leptum |
- |
A significant reduction in HbA1c (P<0.001) |
|
Metformin and Chinese herbal remedy (34) |
Blautia spp. and Faecalibacterium |
- |
FPS, PPPs, and HbA1c significantly improved |
|
whole grains, traditional Chinese medicinal foods, and prebiotics (35) |
Bifidobacterium spp and Bacteroides spp |
|
A better HbA1c |
|
A probiotic supplement consisted of 7 viable strains Lactobacillus, Bifidobacterium, and Streptococcus. (36) |
- |
- |
FPG decreased (P = 0.001) |
|
Probiotic containing Lactobacillus casei (37) |
- |
- |
FPS improved and HbA1c (P=0.013), and 0.077 |
|
A diet low in branched-chain amino acids (38) |
Bacteroidetes |
Firmicutes |
FPS not improved (P=0.17), improved insulin sensitivity |
|
Probiotic containing Scutellaria baicalensis (39) |
Lactobacillus and Akkermansia |
|
Lower glucose tolerance |
