Effect of Functional Ingredients on Viability of Lactobacillus Acidophilus Bacteria in Nondairy Probiotic Products
Roqaya Jaber Asiri 1, Heba Abbas Sindi 1*, Arulazhagan Pugazhendi 2
|
|
|
ABSTRACT
Probiotic bacteria are used in the development of fermented products that can increase the nutritional value of the products. Thus, the aim of this study is to examine the effect of functional ingredients on the viability of L. acidophilus in nondairy products. Four nutritional drinks were prepared including date drink (D), date-coconut drink (DC50), date-oat drink (DO50), and mixture drink (M). The drinks were fermented with 1% of L. acidophilus ATCC 314. The products were analyzed for total fat and protein, pH, titratable acidity, antioxidant activity, sensory evaluation, and viable cell count during storage time at 4 ºC. At the end of the storage period, L. acidophilus was able to survive above 107 CFU/mL in all products, the maximum growth was recorded in D drink which reached 109 CFU/mL, followed by DO50 drink with 108 CFU/mL after 7 days of cold storage. The amount of lactic acid (%) increased in D, DC50, DO50, and M from (0.098, 0.027, 0.035, 0.034) to (0.159, 0.12, 0.138, 0.126) respectively. The increase in lactic acid content led to a reduction in the pH value of the products which confirmed the bacterial growth. The antioxidant activity assay indicated the addition of L. acidophilus to the products increased the antioxidant activity. The sensory evaluation showed that the consumers most preferred D and DC50 in taste and overall acceptability compared to other products DO50 and M. The present research will be used to develop a new nondairy probiotic product at low-cost with high nutritional value.
Keywords: L. acidophilus, probiotic, date, coconut, oat, antioxidant activity
Introduction
Diet plays an important role in ensuring health and preventing diseases. Most diets contain functional components that provide basic nutrition and promote consumers' health. Probiotics are live microorganisms classified as functional components in nutritional food that provide health benefits when consumed. The health benefits of probiotics include the prevention and treatment of infectious diarrhea, constipation, lactose intolerance, and reduction of the symptoms of irritable bowel syndrome [1].
Food applications for probiotics are mostly found in dairy products such as yogurts, kefir, and cheese. The major worldwide importance of nondairy probiotic products is because of the high prevalence of lactose intolerance in numerous populations. Therefore, individuals with this problem avoid dairy products. Nondairy probiotic products have been showing great interest among lactose intolerants and vegetarians. According to the U.S. National Institutes of Health, around 75% of the world population is lactose intolerant. Developing new nondairy probiotic food products is quite challenging, as it must reach the consumers' expectancy for healthy benefits [2, 3]. Technology improved food components to alter structural characteristics of fruits and vegetables by modifying the pH or fortifying culture media which provides an ideal substrate for probiotics culture [2]. Food ingredients such as carbohydrates and fibers have been proven to enhance the viability of probiotics [4].
Dates, coconut, and oat are foods with functional components such as carbohydrates, phenolic, and antioxidant compounds that provide a suitable environment for probiotics [5-7]. Dates are considered a good carbon source for the growth of the probiotic bacteria. In addition, it helps to optimize probiotic Lactobacillus casei production [8]. b-glucan in oat has a prebiotic activity to stimulate the growth of intestinal microflora, with a particular effect on lactic acid bacteria (LAB) [6]. The composition of coconut milk such as carbohydrates and minerals may favor LAB fermentation to produce lactic coconut milk with healthy benefits [9].
Lack of nondairy probiotic products for vegetarians and people allergic to animal proteins or lactose-intolerants reported as a major nutritional issue in Saudi Arabia. The prevalence of lactose intolerance in Saudi Arabia is ranging from (10% - 30%) [10]. Therefore, the development of a nondairy probiotic product is the need for the food industry as an alternative for dairy products. Thus, the aim of the research is to examine the effect of functional ingredients used in the nondairy probiotic products on the viability of Lactobacillus acidophilus bacteria. Also, the study potentially helps people allergic to animal protein or lactose-intolerants and vegetarians.
Materials and Methods
Materials
The ingredients used to make the products were purchased from the local market in Jeddah as following: Date (Khalas Al Qasim) from Oasis Lina dates factory, Saudi Arabia, coconut milk from Orient Provision and Trading Company, Thailand, oat (Walkers Snack Foods Ltd, United Kingdom), freeze-dried L. acidophilus ATCC 314 (American Type Culture Collection, United States), de Man, Rogosa and Sharpe (MRS) agar, MRS broth, Trolox, 2,2-diphenyl-1-picrylhydrazyl (DPPH) and all solvents of analytical assay reagents were obtained from Sigma Aldrich, Germany.
Preparation of date drink
Date drink produced by soaking 25 g of date in 100 ml hot water for 10 min, then blended in a blender mixture, after that the liquid passed through layers of cheesecloth and sterilized at 85 °C for 15 sec, then kept at 4 °C for future use [6].
Preparation of oat drink
Oat milk was prepared as detailed by Bernat et al. [6] with minor modifications. Oat drink produced by soaking 15 g of oat in 100 ml water and blended in a blender mixture, further passed through layers of cheesecloth. The liquid obtained was sterilized at 85 °C for 15 sec.
Experimental design
Four products were prepared to be fermented with L. acidophilus ATCC 314 as the following:
Fermentation process
L. acidophilus ATCC 314 was activated by inoculating the strain in MRS broth and incubated at 30 °C for 48 h. After activation, 1 mL of the cultured MRS broth centrifuged at 4000 rpm for 10 min. cell pellets obtained after centrifugation were washed twice with sterilized saline solution and used to inoculate the product (100 mL). The inoculum was distributed in the product evenly by hand mixing for 30 sec. The inoculated product was then incubated at 37 °C for 3 h to allow fermentation and stored at 4 to 6 °C [11, 12]. The microbial analysis was conducted every 24 hours for 7 days of cold storage.
Chemical, Physical and Microbial Analysis
Sensory evaluation
Nondairy probiotic products were evaluated by 20 consumer panelists using a sensory rating grade according to Bernat et al. [19] with minor modifications, from 1 (very weak) to 5 (excellent) for color, taste, odor, flavor, and overall acceptability.
Statistical evaluation
The statistical evaluation was performed using IBM SPSS software. All experiments were carried out in triplicate, and the results expressed as mean and standard deviation using a one-way analysis of variance (ANOVA) followed by Tukey's test [13, 20].
Results and Discussion
Chemical composition
The total nitrogen and fat of the products are detailed in Table 1. DO50 contained a high amount of total protein (0.23 ± 0.05 %) compared to D, DC50, and M used in the study. The fat content was highest in DC50 with 2.76% compared to other nondairy products. The total fat and protein were found very low in four drinks as all the ingredients are not considered a rich source of fat and protein. The results of fat and protein content were found in agreement with other authors [6, 21-23], which can be used in product commercialization.
The pH value for each product before and after fermentation throughout the 7 days of storage at 4 °C is presented in table (2). The optimum pH for the Lactobacillus acidophilus growth is between 5.5-6.0 [13]. The pH values before fermentation for D, DC50, DO50 and M drinks were 5.88±0.01, 6.33±0.05, 6.22±0.03, and 6.04±0.05 respectively, which is found around the desirable range for the growth of L. acidophilus. After fermentation for D, DC50, DO50, and M drink, the pH decreased to 4.61±0.04, 4.75±0.02, 4.70±0.01, and 4.78±0.04 respectively. These changes in pH value were expected because of the high viability of L. acidophilus during storage time, which may still be producing acidic compounds such as lactic acid. D drink has the lowest pH value which is found similar to the result reported by Karbasi, et al. [20]. The pH value of the DC50 drink of this study was in agreement with results by Edem and Elijah [24]. For DO50 drink the pH value in day 7 was found higher (4.70) in comparison with a previous study by Bernat et al. [6] used oat milk to develop a probiotic product. The change in pH of DO50 may be due to the difference in the components of DO50 which contained 50% of date drink along with oat milk.
Table 1. Total nitrogen and Fat content of fermented products.
|
Sample A |
Protein % |
Fat % |
|
D |
0.09± 0.00 a |
0.26± 0.11 a |
|
DC50 |
0.11± 0.05 a |
2.76± 0.30 b |
|
DO50 |
0.23± 0.05 b |
0.7± 0.17 a |
|
M |
0.14± 0.05 ab |
1.6± 0.30 c |
A D: 100% date drink; DC50: 50% of date drink + 50% of coconut drink; DO50: 50% of date drink+ 50% of oat drink; M: 33.3% of date drink + 33.3% of coconut drink + 33.3% of oat drink.
Means values ± standard deviation.
a,b,c: Different letters in the same column mean significant differences between samples (P<0.05).
Table 2. pH value of non-fermented and fermented drinks.
|
Sample A |
pH (value) Non-fermented |
pH (value) Fermented with L. acidophilus ATCC 314 |
|||||||
|
Day 0 |
Day 1 |
Day 2 |
Day 3 |
Day 4 |
Day 5 |
Day 6 |
Day 7 |
||
|
D |
5.88±0.01a |
5.31±0.06 a |
5.31±0.10a |
5.22±0.03a |
5.13±0.05a |
4.88±0.03a |
4.86±0.02a |
4.78±0.01a |
4.61±0.04a |
|
DC50 |
6.33±0.05b |
5.97±0.05b |
5.87±0.04b |
5.78±0.05b |
5.61±0.03b |
5.36±0.03b |
5.03±0.02b |
4.90±0.05b |
4.75±0.02b |
|
DO50 |
6.22±0.03b |
5.86±0.03b |
5.72±0.03b |
5.66±0.02c |
5.44±0.03c |
5.20±0.02ab |
4.97±0.01c |
4.81±0.01a |
4.70±0.01b |
|
M |
6.04±0.05a |
5.89±0.03b |
5.83±0.02b |
5.80±0.01b |
5.56±0.03b |
5.29±0.02b |
5.01±0.01bc |
4.92±0.02b |
4.78±0.04b |
A D: 100% date drink; DC50: 50% of date drink + 50% of coconut drink; DO50: 50% of date drink+ 50% of oat drink; M: 33.3% of date drink + 33.3% of coconut drink + 33.3% of oat drink.
Means values ± standard deviation.
a,b,c: Different letters in the same column mean significant differences between samples (P<0.05).
Lactic acid bacteria improves the inhibition of the pathogen infection by the production of lactic and acetic acid [25]. In this study, the percentage of lactic acid was measured and tabulated in table 3. The results showed an increase in acidity in all products. The acidity of the D drink averaged 0.098% of lactic acid and increased after fermentation, reaching values up to 0.159%. This result was found in agreement with Karbasi, M. [20]. titratable acidity % of DC50 drink increased from 0.027% to 0.12% which is also in complete agreement with previous reports that used fermented coconut milk and recorded an increase in acidity [24, 26]. DO50 drink reached up to 0.138% after fermentation which is found similar to the result that reported by Bernat et al. [6]. For the M drink, the total amount of lactic acid was 0.126% after fermentation. This elevation of lactic acid occurs due to the presence of L. acidophilus that generated carbohydrates to produce lactic acid.
Table 3. Titratable acidity % lactic acid in non-fermented and fermented drinks.
|
Sample A |
Non-fermented (TA % lactic) |
Fermented with L.acidophilus ATCC 314 (TA % lactic) |
|
D |
0.098± 0.01 a |
0.159±0.01 a |
|
DC50 |
0.027±0.00 b |
0.12±0.01 b |
|
DO50 |
0.035±0.01 b |
0.138±0.01 c |
|
M |
0.034±0.01 b |
0.126±0.00 bc |
A D: 100% date drink; DC50: 50% of date drink + 50% of coconut drink; DO50: 50% of date drink+ 50% of oat drink; M: 33.3% of date drink + 33.3% of coconut drink + 33.3% of oat drink.
Means values ± standard deviation.
a,b,c: Different letters in the same column mean significant differences between samples (P<0.05).
Antioxidant activity of non-fermented and fermented products
The antioxidant activity by DPPH and TEAC assay was detailed in Tables 4 and 5. The DPPH assay is based on the assessment of the antioxidants' scavenging capacity. The electron of the nitrogen atom in DPPH is decreased due to obtaining a hydrogen atom from an antioxidant [27]. In the current study, all four products showed an elevation in antioxidant activity after fermentation and these results were in complete agreement with previous studies that measured the effect of adding bacteria in increasing the antioxidant activity of food [11, 20]. DPPH assay results revealed D drink possesses significantly higher antioxidant activity compared to other drinks. This increase may be due to the presence of cinnamic acid and its derivatives responsible for the antioxidant activity of date fruit. Also due to its structure, cinnamic acid is considered a good hydrogen donor [7, 20]. A research carried out by Jayabalan et al. [28] showed that the presence of β-glucosidase enzyme in lactic acid bacteria may enable the release of the phenolics that are bound with sugars which can lead to the increment of the antioxidant activity of food.
Table 4. Antioxidant activity DPPH % in non-fermented and fermented products
|
Sample A |
Non-fermented (DPPH %) |
Fermented with L.acidophilus ATCC 314 (DPPH %) |
|
D |
53.85 ±0.47 a |
57.49 ±0.28 a |
|
DC50 |
14.6±0.49 b |
18.25±0.37 b |
|
DO50 |
23.39±0.45 c |
31.21±0.45 c |
|
M |
12.23±0.34 d |
16.07±0.22 d |
A D: 100% date drink; DC50: 50% of date drink + 50% of coconut drink; DO50: 50% of date drink+ 50% of oat drink; M: 33.3% of date drink + 33.3% of coconut drink + 33.3% of oat drink.
Means values ± standard deviation.
a,b,c: Different letters in the same column mean significant differences between samples (P<0.05).
Table 5. Antioxidant activity TEAC for non-fermented and fermented products (mg Trolox/mL)
|
Sample A |
Non-fermented (mg Trolox/mL) |
Fermented with L.acidophilus ATCC 314 (mg Trolox/mL) |
|
D |
2.80±0.40 a |
4.30± 0.17 a |
|
DC50 |
4.41±0.31 bc |
5.27± 0.43 bc |
|
DO50 |
5.22±0.26 c |
6.05± 0.51 c |
|
M |
3.72±0.56 ab |
4.35± 0.26 ab |
A D: 100% date drink; DC50: 50% of date drink + 50% of coconut drink; DO50: 50% of date drink+ 50% of oat drink; M: 33.3% of date drink + 33.3% of coconut drink + 33.3% of oat drink.
Means values ± standard deviation.
a,b,c: Different letters in the same column mean significant differences between samples (P<0.05).
Enumeration of Lactobacillus Acidophilus
The growth pattern of L. acidophilus ATCC 314 in nondairy drinks throughout the storage period of 7 days at 4 °C was illustrated in Figure 1. Lactobacillus acidophilus attained a high cell population and survived above the recommended value of 106 CFU/mL during cold storage. In the present study, all four products had bacterial growth levels above 106 CFU/mL, L. acidophilus recorded a maximum growth in D drink which reached 109 CFU/mL after 7 days of cold storage. The maximum growth may be due to the presence of high simple sugar content in date compare to other products that stimulate the growth of the bacteria. Karbasi et al. [20] reported that Lactobacillus acidophilus used in date syrup exhibited potential probiotic activity. DO50 drink showed high viability of L. acidophilus due to the prebiotic effect of oat that enhances the growth of the bacteria. This result was found similar to Bernat et al. [6] studied the viability of certain strains in oat milk.
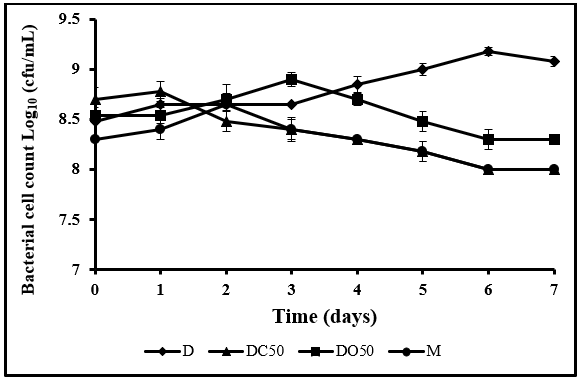
Figure 1: Cell count of L. acidophilus ATCC 314 in nondairy drinks throughout 7 days of storage at 4 ℃. D:
100% date drink; DC50: 50% of date drink + 50% of coconut drink; DO50: 50% of date drink+ 50% of oat drink; M: 33.3% of date drink + 33.3% of coconut drink + 33.3% of oat drink.
Means values ± standard deviation.
Another report by Kurtuldu et al. [29] found that the viability of the bacteria was significantly enhanced by b-glucan supplementation which is found naturally in oat. For DC50 and M drinks, the growth rate was lower compare to D and DO50 drinks but remained within a desirable range (107 CFU/mL) after 7 days of cold storage, and these findings were in agreement with previous researches [9, 26], examined the effect of coconut milk on the viability of probiotic to be a nondairy probiotic product.
Sensory evaluation
The results in Table 6 showed the sensory evaluation of D, DC50, DO50, and M drinks. D and DC50 drinks reached higher scores in color, taste, odor, flavor, and overall acceptability. Most people prefer the sweetness in food and the taste of date fruit, especially in Saudi Arabia. DC50 was preferred by the panelists due to its strong pleasant flavor and this was in agreement with other research studies [23, 26] evaluated the sensory of coconut milk in developing a new nondairy probiotic product. DO50 contained least preferred in taste, color, flavor, and overall acceptability and M drink as well since it contained 33% of oat drink.
Table 6. scores from 5 to 1 of sensory evaluation of fermented nondairy drinks.
|
Sample A |
Color |
Taste |
Odor |
Flavor |
Overall acceptability |
|
D |
4.40 a |
4.65 a |
4.60 a |
4.60 a |
4.50 a |
|
DC50 |
4.25 a |
3.95 ab |
4.35 a |
3.85 ab |
3.75 ab |
|
DO50 |
3.70 a |
3.20 b |
3.90 a |
3.15 b |
3.35 b |
|
M |
3.85 a |
3.35 b |
3.85 a |
3.35 b |
3.45 b |
A D: 100% date drink; DC50: 50% of date drink + 50% of coconut drink; DO50: 50% of date drink+ 50% of oat drink; M: 33.3% of date drink + 33.3% of coconut drink + 33.3% of oat drink.
Means values ± standard deviation.
a,b,c: Different letters in the same column mean significant differences between samples (P<0.05).
Conclusion
The research findings of the study highlight the beneficial effect of the functional ingredients on the viability of the bacteria which was able to survive above the recommended value due to the high presence of carbohydrates that stimulate the growth of the bacteria that found the highest in D drink followed by DO50. The high viability of L. acidophilus led to an elevation of lactic acid and a reduction in pH value. The results of this study proved that L. acidophilus enhanced the antioxidant activity of the drinks. Moreover, the sensory evaluation showed that D and DC50 drinks were the most acceptable by the consumers for their sweetness and strong pleasant flavor. Therefore, this study revealed the potential for the development of a new nondairy probiotic product with nutritional benefits for vegetarians, lactose intolerants, or those allergic to animal proteins.
Acknowledgment:
The authors thank Prof. Iqbal Mohammad Ibrahim Ismail, Director, Center of Excellence in Environmental Studies, King Abdulaziz University, Jeddah, Saudi Arabia for his extensive support for providing sources required for the research.
Conflict of interest: The authors declare that they have no conflict of interest.
References
