NEUROPROTECTIVE EFFECT OF VIRGIN COCONUT OIL AGAINST HYDROCARBON INDUCED NEUROTOXICITY
Aysha A. Alshareef 1*, Maha Ibrahim 2
|
|
|
ABSTRACT
There is conscious and subconscious exposure to the harmful effect of hydrocarbon like benzene daily; be it from cigarette smoke, vapor from a service station, and exhaust from moving vehicle. The toxicity of benzene exposure affects both the immune and central nervous system and this has been proposed to be due to the oxygen-free radicals liberated from benzene metabolism with a potentially damaging effect on biomolecules. In this study, we investigated the potentials of virgin coconut oil (VCO), a natural source of antioxidant to protect against neurotoxicity caused by exposure to benzene inhalation in rats. Rats were exposed to 10ppm of benzene for two hours thereafter administered with an oral dose of 600mg/kg body weight VCO. Benzene exposure resulted in increased lipid peroxidation, a decrease in the activity of superoxide dismutase, and reduced glutathione levels in brain tissues. Also, benzene exposure resulted in increased serum levels of urea, creatinine, and uric acid. However, treatment with VCO significantly ameliorated these effects. This study showed that VCO has neuroprotective property against benzene toxicity and has the potential to prevent renal dysfunction.
Keywords: Antioxidant, Benzene, Hydrocarbon, VCO
Introduction
Benzene, an aromatic hydrocarbon has found wide usage in the industrial production of resins, polymers, and fibers. It is a common constituent of gasoline, wood and cigarette smoke. Day to day exposure to benzene is thought to occur through the inhalation of cigarette smoke, vapors in service stations, or by the exhausts released from moving vehicles. Industrially, exposure to benzene is seen in oil refineries, printing factories and factories dealing with rubber and shoe production [1]. Benzene is widely used as a solvent in different industries [2-4].
In humans, an estimated amount of 50% of inhaled benzene is absorbed and this amount is dependent on its concentration and duration of exposure [5]. Once absorbed, benzene is metabolized by various organs into reactive intermediate such as hydroquinone, benzene oxide, benzoquinones, phenol and catechol some of which have been implicated in the production of reactive oxygen species [6] with potentials to damage biomolecules such as DNA, RNA, and proteins [7]. For example, when benzene is metabolized into phenol, it resulted in the peroxidation of arachidonic acid [8].
Fresh coconut palm (Cocos Nucifera L.) was used to extract extra virgin oil which was not processed by heat or undergone chemical refining. It is made up of carbohydrates, polyphenols, dietary fibers, and minerals [9]. It also contains phenolic acids such as ferulic acids, catechin p-Coumaric acid [10]. Virgin coconut oil (VCO) has been reported to possess anti-inflammatory, antioxidant, anti-stress [11], and anti-diabetic properties [12].
In this study, we investigated the potentials of VCO to protect against neurotoxicity caused by exposure to benzene inhalation in rats
Materials and Methods
Animals
Male Wistar rats (n=40) weighing between 180and 200 g were obtained from King Fahd Medical Centre, KAU, SA. The rats were fed on a normal chow diet with access to water and housed at the Preclinical unit, King Fahd Medical Centre, King Abdulaziz University, and allowed to acclimatized at an ambient temperature of 22±2°C and 12 hr dark/12 hrs light cycle for one week.
The rats were then randomly divided into 4 groups with 10 rats per group as follows:
GRP1 (control rats): unexposed and untreated rats. Rats were maintained on normal chow diet and water.
GRP 2: rats were exposed to daily 10ppm of benzene for 2hrs in an exposure chamber (75 x25 x 40 cm) after which they were moved to their normal cages and allowed to free normal food and water.
GRP 3: rats were exposed to daily 10ppm of benzene for 2hrs in an exposure chamber (75 x25 x 40 cm) after which they were moved to their normal cages and allowed to free normal food and water.600 mg/kg/day of VCO was then administered to this group.
GRP 4:rats were maintained on normal chow diet and water and received 600 mg/kg/day of VCO.
After 30 days, rats were fasted overnight and euthanized using diethyl ether. Blood was withdrawn from the abdominal aortal and brain tissues were removed and stored at -80°C until analysis.
All procedures involving lab animals were conducted following the guideline of the US National Institutes of Health Guidelines for Care and Use of Laboratory Animals in Biomedical Research (2001).
Extraction of VCO
Mature coconut was obtained from a local market and VCO was extracted according to the method of Nevin and Rajamohan (2004) [13]. Briefly, the coconut was crushed, made into a viscous slurry, and squeezed through cheesecloth. The coconut milk obtained was refrigerated for 2 days and was mildly heated at 50° to obtain the virgin oil.
Preparation of brain homogenate
The brain was homogenized using a 100 mM phosphate buffer solution, pH = 7.4. The supernatant was separated after half-hour centrifugation at 3000 rpm at 4 ° C. The supernatant was collected and stored at -80°C until analysis.
The level of superoxide dismutase (SOD) and dopamine level in the brain homogenate was determined using superoxide dismutase activity colorimetric assay Kit and dopamine (DA) ELISA Kit (BioVision, USA), respectively according to the manufacture’s instruction.
Determination of brain lipid peroxidation and glutathione (GSH) level
Lipid peroxidation extent was assessed in the brain homogenate according to the method of Rehncrona et al., [14] using thiobarbituric acid (TBA) as a reagent. The absorbance of the colored malondialdehyde (MDA) complex formed was determined at 532nm against reagent blank. The MDA content was expressed as nmol/ml.
GSH concentration was assessed in the brain homogenate according to Moron et al. [15]. Sodium phosphate buffer (900 µl, 0.2 M, pH = 8.0), as well as DTNB (5,5'-dithiobis nitro benzoic acid, 2000 µl), were added to 100 µl of brain homogenate. Within 10 min, the absorbance of the formed yellow color was quantified with the spectrometer at 412 nm.
Determination of serum level of uric acid, urea, and creatinine
To obtain serum, whole blood was centrifuged at 3000 rpm for 10 min at 4ºC. The serum level of kidney function biomarkers; uric acid, urea, and creatinine were determined using a commercial kit (BioVision, USA) according to the manufacturer’s instructions.
Statistical analysis
Data are reported as mean ± SEM. Statistical analysis using one-way ANOVA with Turkey’s multiple comparison test and figures were presented employing GraphPad Software (version 6), La Jolla, CA, USA. The level of significance was kept at p < 0.05.
Results
Effect of benzene and VCO on brain SOD and dopamine
The level of the antioxidant enzyme SOD and the neurotransmitter dopamine were significantly decreased by the inhalation of benzene in untreated rats exposed to benzene as compared to the control rats not exposed to benzene (Fig 1). The administration of VCO post-benzene inhalation significantly prevented the superoxide radical-induced oxidative stress and decrease in dopamine levels in the rat brain. Rats treated with only 600mg/kg body weight VCO showed similar levels of SOD and dopamine when compared to untreated control rats (Fig 1).
Figure 1: Effect of benzene and VCOon brain SOD and dopamine level. SOD level (A), dopamine level (B).
Effect of benzene and VCO on MDA and GSH in brain
The inhalation of benzene by rats resulted in the induction of oxidative stress in rat brain as evidenced by the increase in lipid peroxidation (MDA level) and a decrease in the concentration of reduced glutathione (GSH) when compared to animals in the control group (Fig 2). Coconut oil (600mg/kg) administered post-benzene inhalation resulted in a significant decrease in lipid peroxidation while also boosting the brain GSH formation relative to untreated animals exposed to benzene inhalation(Fig 2). Also, rats treated with only oral 600mg/kg bodyweight of VCO showed similar levels of SOD and dopamine when compared to untreated control rats (Fig 2).
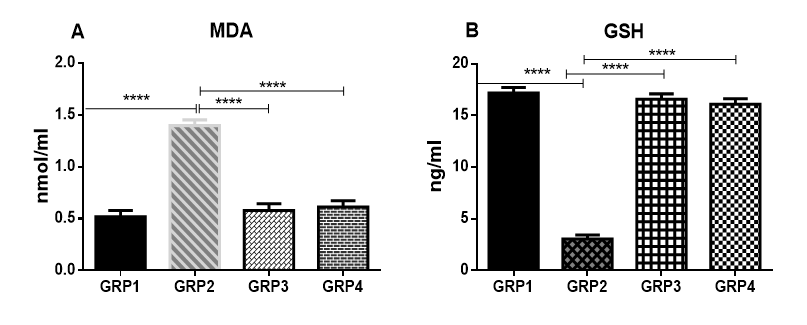
Figure 2: Effect of benzene and VCOon MDA and GSHconcentration in the brain. MDA (A), GSH (B).
Effects of benzene and VCO on serum renal function in rats
The nephrotoxic effect of benzene inhalation is rat was obvious by the significant increases in serum levels of urea, uric acid, and creatinine when compared to rats in the control group not exposed to benzene (Fig 3). The 600mg/kg VCO separately and post-inhalation administered VCO attenuated the nephrotoxicity induced by benzene and restored the levels of these serum biomarkers to normal levels when compared to untreated animals in the control group not exposed to benzene inhalation (Fig 3).
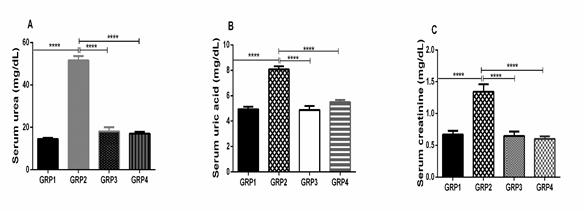
Figure 3: Effects of benzene and VCOon serum renal function in rats. Urea (A), uric acid (B), and creatinine (C) levels.
Discussion
The brain is an indispensable tissue that consumes a considerable amount of oxygen for its metabolic processes. Hence maintaining the brain in its biochemical form is crucial not only for neuron’s survival but also for its development and the normal functioning of the central nervous system (CNS) [16]. The brain by its nature consists of a considerable amount of polyunsaturated fatty acid (PUFA) and a minimal amount of antioxidant defense system coupled with its high oxygen demand. This setting makes the brain susceptible to oxidative stress.
Oxidative stress occurs when the ability of the antioxidant defense system in the brain is overwhelmed by the excess production of free radicals [16]. Previous studies have shown that different neurogenerative diseases like amyotrophic lateral sclerosis (ALS), Parkinson’s disease, and Alzheimer's disease are caused by oxidative stress that affects the normal function of the mitochondria [17].
However, in protecting itself from the insults emanating from ROS, the brain utilizes its radical scavenging defense system such as SOD, catalase, and glutathione reductase in addition to the use of low-molecular-weight antioxidants like glutathione to counteract any potential damage [6]. Glutathione in its reduced GSH form is the most indispensable nonenzymatic antioxidant naturally occurring in tissues [18].
In this study, we investigated the potential neuroprotective effect of coconut oil against neurotoxicity caused by exposure to benzene inhalation in rats. Our results showed that exposure to benzene results in the decrease in the superoxide dismutase level, elevation in lipid peroxidation and decrease in the reduced glutathione (GSH) level in the brain of rats; a situation of oxidative stress. Our data are similar to former research that showed an increase in lipid peroxidation quantified as MDA [19] and a decrease in the SOD level [20] in humans exposed to organic solvent including benzene. According to Georgieva et al. (2002) [21], benzene exposure caused disequilibrium in the antioxidant level and ROS formation as a result of oxidation arising from benzene metabolites such as hydroquinone and 1,2,4-benzenetriol. However, the administration of VCO following benzene exposure restored the SOD level, decrease MDA, and increased GSH formation in the brain.
Studies have shown that coconut oil is enriched with flavonoids and polyphenols that can counteract free radical responsible for ROS generation in the cell [10]. These polyphenols have neuroprotective effects seen in the prevention of neurotoxicity of β-amyloid [22].
The decrease in the level of dopamine serves as a diagnostic biomarker for Parkinson’s disease. The damage of dopaminergic nerve cells results in a decline in the level of dopamine [23]. Our results showed that exposure of rats to benzene caused a reduction in the concentration of the brain dopamine. However, treatment with VCO restored this level (Fig 1). The administration of VCO after benzene exposure likely resulted in a decrease in dopamine metabolism or an increase in its biosynthesis by the dopaminergic neurons present in the substantia nigra of the brain [23].
Furthermore, the plasma concentrations of urea and creatinine are biomarkers of renal function. An elevation in their serum levels serves as an indication of impaired kidney function [24]. More so, elevated levels of serum uric acid may signal a dysfunctional kidney and may indicate an underlying cardio-metabolic problem [25].
Our results, however, showed that exposure to benzene produced a marked rise in the serum concentration of urea and creatinine and uric acid (Fig 3). This result is in per with previous reports which showed that exposure to hydrocarbon leads to an increased serum urea creatinine and uric acid [26]. Treatment with VCO after benzene exposure restored the levels of these biomarkers to normal.
In conclusion, we report here, the potential neuroprotective effect of VCO against neurotoxicity induced by exposure to benzene in rats. We showed that benzene exposure resulted in oxidative stress as quantified from the increased lipid peroxidation, decrease in SOD and GSH levels. We also showed that exposure to benzene impaired renal functions. Treatment with VCO post-exposure of benzene, however, counteracted its harmful effect. This study showed that VCO has neuroprotective property against benzene toxicity and has the potential to prevent renal dysfunction.
References
