Valsartan: A Brief Current Review
Azman Abdullah1*, Muhammad Fadzli Rusli2
|
|
|
ABSTRACT
Hypertension is defined as having blood pressure exceeding the normal range of 120/80 mmHg. One of the more recent drugs to treat hypertension is valsartan, which belongs to the Angiotensin-receptor blockers (ARB) group of drugs. Since its introduction in 1996, it has been widely used to treat hypertension due to its efficacy and being well-tolerated by patients. In this brief review, an update of all pharmacological aspects of valsartan will be succinctly discussed, which includes pharmacokinetics, pharmacodynamics, indications, contraindications, adverse effects, and drug interactions. A brief comparison between valsartan and losartan is also attempted in this review.
Keywords: valsartan, angiotensin-receptor blockers, hypertension, pharmacology
Introduction
Hypertension is a multifactorial medical condition which essentially described as persistently high blood pressure exceeding the normal range of blood pressure which is set at systolic over diastolic at 120/80 mmHg. This condition can progress to heart disease which if not properly controlled, will lead to heart failure. According to the World Health Organization (WHO), heart failure (HF) is the current leader of the premature death of non-communicable diseases in South-East Asia [1]. Unfortunately, due to what people’s lifestyle has evolved, or perhaps regressed into, it is becoming increasingly more prevalent in many parts of the world [2-4]. As the most obese nation in the South-East Asia region, Malaysians are at high risk of developing HF. Depending on the severity of the condition, pharmacological and non-pharmacological approaches may be deployed. Non-pharmacological approaches are mainly focused on lowering the risk of developing heart disease and controlling blood pressure in individuals with pre-hypertensive conditions [5]. This form of intervention may include management in dietary intake, increased physical activity, and cessation of smoking and alcohol intake. On the other hand, pharmacological approaches require the consumption of antihypertensive drugs to control blood pressure in individuals that are no longer responsive to non-pharmacological interventions.
The current pharmacological intervention involves the treatment of hypertensive patients with different classes of drugs such as angiotensin-converting enzyme inhibitors (ACEI), α and β-blockers, diuretics, angiotensin-receptor blockers (ARB), and calcium channels blockers (CCB), among others [6]. Newly diagnosed hypertensive patients and uncomplicated cases with no compelling indications may be prescribed with only one class of antihypertensive drugs. In some cases, a combination of therapy is required and may be instituted early especially in patients at stage II hypertension and high-risk stage I hypertension. Valsartan is classified under ARB drugs. Two formulations of valsartan are available in Malaysia, including the single active compound (i.e. valsartan only formulation), and the other is a combination of valsartan with one or more active ingredients e.g. amlodipine besylate, and hydrochlorothiazide [6]. The aim of this current review is to briefly discuss the pharmacology of valsartan.
Brief pharmaceutical aspects of valsartan
Valsartan is chemically described as N-(1-oxopentyl)-N-[[2’-(1H-tetrazol-5-yl) [1,1’-biphenyl]-4-yl]methyl]-L-valine (Figure 1). Its empirical formula is C24H29N5O3 with a molecular weight of 435.5 grams per mole. It exists in physical form as a white powder. Valsartan is soluble in alcohol solvents such as ethanol and methanol, and only slightly soluble in water. It is commercially available in tablet form with the active compound mixed with inactive ingredients such as colloidal silicon dioxide, crospovidone, iron oxides, magnesium stearate, hydroxypropyl methylcellulose, microcrystalline cellulose, polyethylene glycol 8000, and titanium dioxide. Valsartan also shows pH-depending solubility, where it shows high solubility in pH 5-8 but decreased solubility in more acidic pH [7].
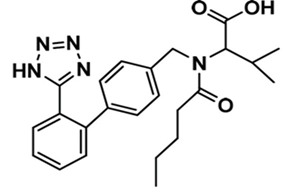
Figure 1: Chemical structure of valsartan
Pharmacokinetics of valsartan
The pharmacokinetics of drugs can be classified into four processes which are absorption, distribution, metabolism, and elimination. The pharmacokinetics of valsartan will be expressed below based on the processes.
a) Absorption
Valsartan is quickly absorbed post-oral administration. After oral administration of 80 mg valsartan in capsule and solution formulation in twelve healthy volunteers, maximum plasma concentrations (Cmax) of Valsartan (1.64mg/l and 3.25 mg/l) were respectively achieved within 1 to 2 hours. Plasma levels and the area under the plasma concentration-time curve (AUC) were not linearly correlated with dose, demonstrating a saturable first-pass metabolism [8]. The absolute bioavailability of valsartan is 23% and 39% for capsule and the solution formulation respectively. Deconvolution techniques showed that the average amount of valsartan entering the systemic circulation was 24% from the capsule and 41% from the solution formulation. 50% of valsartan in capsule formulation was absorbed by 1.6 hours, and 90% by 4.6 hours. Valsartan solution formulation was absorbed faster, in which 50% of the drug was absorbed and was available in systemic only within 0.8 hours, and 90% within 2.4 hours [8]. Additionally, the Cmax of Valsartan increased two-fold (3.95 mg/l) in patients with heart failure after an oral administration of 80mg Valsartan capsule [9]. Food has not been recorded to cause any effect on drug absorption. Thus, valsartan can be consumed with or without food [10].
b) Distribution
Valsartan is highly bound to plasma protein (94-97%), and the main plasma protein involved is albumin (92%). This causes limitations to its distribution outside the plasma compartment. The volume of distribution at steady state is 17 L, which is less than the total body water volume. This further suggests the lack of distribution of valsartan to extravascular tissues [8, 11]. Valsartan also shows pH-dependent solubility. It is soluble in a neutral pH range and is mainly present in the ionized form at physiological pH due to the presence of carboxylic groups. The in vitro test of Valsartan showed rapid and complete dissolution at pH 5-8 and limited solubility at pH 3 and lower [12].
c) Metabolism & elimination
Valsartan is readily active, and prior metabolism of the parent drug is not required. The primary metabolite of valsartan was determined as valeryl 4-hydroxy valsartan. Only a small portion (11%) of valsartan is metabolized after an oral administration of 80 mg of 14C-radiolabelled valsartan, which was further determined to be pharmacologically irrelevant due to very low affinity of the metabolite for the AT1 receptor as compared to valsartan [13]. The enzyme responsible for valsartan metabolism is yet to be clarified, but CYP450 isozymes are unlikely. Following intravenous administration, plasma clearance and renal clearance of valsartan are respectively about 2 L/h and 0.62 L/h (about 30% of total clearance) [10].
Valsartan is mostly eliminated in stool through the biliary excretion pathway, and only minimally excreted by the renal route. More than 80% of the dose is recovered in feces after a single administration of a valsartan oral solution, while the remaining is found in the urine [13, 14]. The recovery is mainly as unchanged drug, with only about 20% of the dose recovered in stool as metabolites [14]. Dose adjustment is not needed regardless of age. A previous study performed on an elderly population with an average age of 72 showed higher systemic exposure of valsartan when compared to the younger population with an average age of 23. However, the findings of the study did not warrant any alteration of the initial dose [15]. In another study in which the average age is of the patient 70, plasma concentration generally falls by 22% compared with a younger population with an average age of 55. However, the study did not suggest any requirement for dose reduction in this particular group [16]. Although there are differences in the pharmacokinetics of valsartan in patients with heart failure, no changes in dosage are required [9, 17].
Pharmacodynamics of valsartan
Angiotensin-receptor blockers (ARB), the drug group in which valsartan belongs to, generally disrupts the Renin-Angiotensin-Aldosterone System (RAAS) by competing with angiotensin II binding at type-1 angiotensin II receptor (AT1), thus preventing the cellular actions of angiotensin II which include vasoconstriction, cellular proliferation, as well as cytokines and aldosterone production (Figure 2) [18, 19]. Since valsartan has a 20,000-fold greater affinity for the AT1 receptor rather than the type-2 angiotensin II receptor (AT2), the AT2 receptor may be exposed to higher concentrations of Angiotensin II produced by the negative feedback of the RAAS. However, the significance of AT2 receptor activation is not fully understood [7, 19]. The RAAS under normal circumstances helps to regulate sodium and water intake of the body. However, when over-activated, the RAAS contributes to the development of hypertension and the initiation of a molecular action in tissues which will result in the injury of critical organs such as the heart, brain, kidneys, and blood vessels. The initial metabolite of valsartan is essentially inactive with a very low affinity towards the AT1 receptor [13]. Investigations have shown that valsartan alone and in combination with other drugs from different classes reduced hypertension and improved mortality and morbidity in patients [18].
The barricade of the renin-angiotensin system with ACE inhibitors, that prevent the biosynthesis of angiotensin II from angiotensin I, is prevalently employed in the remedy of hypertension (Figure 2). ACE inhibitors also prevent the degradation of bradykinin, a reaction also catalyzed by ACE (Figure 2). As valsartan does not repress ACE (kininase II), it does not modify the response to bradykinin. Whether this variation has clinical significance is not yet proved. Valsartan does not adhere to or block other hormone receptors or ion channels revealed to be important in cardiovascular management. The barricade of the angiotensin II receptor prevents the negative regulatory feedback of angiotensin II on renin secretion, but the resulting improved plasma renin activity and angiotensin II circulating levels do not overcome the impact of valsartan on blood pressure. This will then decrease the blood pressure to a notable level [20].
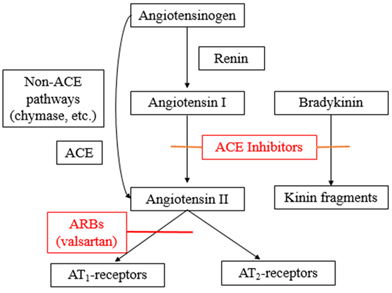
Figure 2: Mechanism of action of valsartan.
Indications of valsartan
Valsartan largely indicated for the treatment of hypertension i.e. to lower and control the blood pressure which ultimately reduces the risk of unfavorable cardiovascular events, primarily stroke and myocardial infarction. Benefits of valsartan in managing hypertension cases have been supported by VALUE trials, in which valsartan-treated patients indicated a reduction of blood pressure and decrease in cardiovascular morbidity and mortality [21]. Apart from primary hypertension, this drug is also reported for the treatment of heart failure and post-myocardial infarction (MI).
In a randomized, double-blind, placebo-controlled investigation called Val-HeFT, valsartan treatment demonstrated a significant decrease in mortality and morbidity for patients with heart failure [22]. There is no indication that valsartan provides additional advantages when it is utilized together with enough doses of an ACE inhibitor. Generally, valsartan (and also other ARB) is a more efficient inhibitor of the renin-angiotensin-aldosterone system than the ACE inhibitors. Valsartan appears to be better tolerated in the context of side effects such as cough and angioedema, which is particularly seen during ACE inhibitors therapy [22].
The VALIANT research, which was performed on patients with left ventricular systolic dysfunction, heart failure, or both following an acute myocardial infarction, compared the efficacy and safety of long-term treatment with valsartan, captopril and their combination in 14,703 high-risk patients after MI. It is a multicentre, double-blind, randomized, active-controlled, parallel-group investigation [23]. The result of the study showed that there is no difference in mortality among patients being treated with captopril 50 mg, valsartan 160 mg, or the combination of valsartan 80 mg with captopril 50 mg. Since the result of the study found no difference between valsartan and captopril therapy, it was suggested that valsartan is suitable to be indicated for the treatment of post-MI [23].
Contraindications and precautions
Valsartan is contraindicated in patients with underlying hypovolemic conditions. Consumption of valsartan under this condition may lead to symptomatic hypotension and can be life-threatening. Due to its pharmacological activity, valsartan in solution formula should only be reserved for the patient with the inability to consume the tablet. Valsartan’s peak plasma concentration is high after the administration of the oral solution and possessed a higher risk of symptomatic hypotension compared to tablets. In the VALIANT research, permanent discontinuation of therapy was issued to 1.4% of valsartan-treated patients and 0.8% of captopril-treated patients because of hypotension in patients with post-myocardial infarction [24]. Valsartan is not contraindicated in transient hypotension but stabilization of blood pressure is needed before the continuation of therapy.
Comparably, in patients with severe hepatic disease, valsartan is contraindicated. Patients with mild to moderate hepatic impairment, such as those with biliary obstruction, have notably improved plasma valsartan concentrations in comparison with patients with normal hepatic function. Although no specific primary dosage adjustment is suggested, caution must be exercised when valsartan is administered to these patients. Dosage elevation should be done cautiously. In addition, valsartan is mainly excreted in feces by biliary excretion and is not suggested for patients with hepatic dysfunction and biliary cirrhosis [25].
There is no specific study that has been conducted to evaluate the use of valsartan in pregnancy and breastfeeding. However, ARB and ACEI are similar as both of them influence the renin-angiotensin system. These drugs may decrease foetal renal function and enhance foetal and neonatal morbidity and death if administered during pregnancy. There are conflicting data about this matter. Retrospective data revealed that the usage of ACE inhibitors in the first trimester has been correlated with a potential risk of birth defects [26]. Despite this data, the investigation did not take into account any pre-existing conditions e.g. maternal hypertension and diabetes which may be responsible for fetal malformation and thus make it even harder to distinguish the effect of the drug [27, 28]. Furthermore, a cohort study of over 1.3 million patients showed no significant correlation between the consumption of hypertensive drugs in the first trimester and foetal malformation [29]. Breastfeeding is not a problem when taking ACEIs. Some ACEIs are drugs of choice to treat hypertension during lactation. Therefore, the same principle may be applied when using valsartan.
Not much data is available on the effect of valsartan in ARB-induced angioedema. However, there is a report that implied valsartan may induce angioedema during the surgical operation. The patient had been taking valsartan (160 mg) for 4 years and was reported to have severe swelling in the neck and the face with edema in his mouth area (tongue, the floor of the mouth, glottis, and supraglottic areas) [30]. The link and precise mechanisms of valsartan-induced angioedema are still unclear. Therefore, patients should be thoroughly investigated and warrant a precaution in the side effects of ARB consumption before surgery.
Adverse effects of valsartan
In general, the previous studies indicated that valsartan was well tolerated. It has been assessed for its safety in 5010 patients with heart failure of New York Heart Association (NYHA) class II, III, or IV. In this cohort, 249 patients receiving valsartan in comparison with 181 patients receiving placebo demonstrated adverse effects including hypotension, dizziness, and renal impairment which cause termination of therapy [31]. Similarly in the Val-HeFT study, 18 out of 185 patients received valsartan showed adverse effects but the number is not significant. Valsartan has been assessed for safety in more than 4000 patients, including over 400 treated for over 6 months, and more than 160 for over 1 year. Adverse reactions have usually been mild and transient and have only uncommonly needed discontinuation of therapy. The overall occurrence of adverse reactions with valsartan was comparable with the placebo. The overall prevalence of adverse reactions was neither dose-dependent nor associated with gender, age, race, or regimen. Discontinuation of therapy because of side effects was needed in 2.3% of valsartan patients and 2% of placebo patients. Dizziness and headache were the most common reasons for discontinuation of therapy with valsartan [32].
Adverse reactions that happened at a higher incidence in valsartan-treated patients in comparison with placebo-treated patients include viral infection (3% vs. 2%), fatigue (2% vs. 1%), and abdominal pain (2% vs. 1%). Headache, dizziness, upper respiratory infection, cough, diarrhea, rhinitis, sinusitis, nausea, pharyngitis, edema, and arthralgia occurred at about the same incidence in placebo and valsartan patients. Dose-related orthostatic influences were revealed in less than 1% of patients. An increase in the incidence of dizziness was observed in patients treated with valsartan 320 mg (8%) compared to 10 to 160 mg (2% to 4%) [7, 32]. Valsartan has been utilized concomitantly with hydrochlorothiazide without indications of clinically important adverse interactions [25]. As for patients with heart failure, the profile of the adverse impact of valsartan in heart failure patients was in line with the pharmacology of the drug, and the health conditions of the patients. In the Valsartan Heart Failure Trial, 10% of valsartan patients discontinued for adverse reactions, in comparison with 7% of placebo patients [22].
Drug interactions
No clinically notable pharmacokinetic interactions were indicated when valsartan tablets were coadministered with hydrochlorothiazide, cimetidine, amlodipine, atenolol, furosemide, digoxin, glyburide, or indomethacin [33]. The valsartan-atenolol combination was more antihypertensive than either component, but it did not decline the heart rate more than atenolol alone [34]. Coadministration of valsartan and warfarin did not modify the pharmacokinetics of valsartan or the time-course of the anticoagulant features of warfarin [35]. There is no indication that valsartan has additional advantages when it is utilized with a suitable dose of an ACE inhibitor. Generally, ARBs like valsartan are more efficient inhibitors of the renin-angiotensin-aldosterone system than ACE inhibitors. Valsartan appears to be better tolerated in the context of side effects e.g. cough and angioedema, which is experienced by patients receiving ACE inhibitors. Valsartan is not recommended to be used in combination with ACE inhibitors due to unfavorable outcomes. A posthoc analysis of the Val-HeFT trial had shown that the combined therapy with valsartan, an ACEI, and a beta-blocker is associated with an increased risk of heart failure morbidity and total mortality [22]. The VALIANT investigation has also reported that the combination of valsartan with an ACEI (captopril) enhanced the rate of adverse events without improving survival [24].
Comparison between valsartan and losartan
Another drug that is in the same class with valsartan is losartan (an ARB). Losartan, the first of the angiotensin II receptor blockers (ARBs) to be introduced, has been investigated prevalently in comparison with other classes of antihypertensive agents. Losartan differs from other ARBs such as valsartan and irbesartan as it has a shorter duration of the function. Nevertheless, the influence of losartan taken once daily at lower doses (25 or 50 mg) does not last the full 24 hours. Only a higher daily dose (100 mg) of losartan is efficient for the full 24 hours. In contrast, valsartan at the higher dose can also provide full 24-hour coverage with once-daily dosing, but with a better response rate than losartan. Losartan and valsartan are both comparably well tolerated by patients. Losartan and valsartan are comparably efficient in decreasing blood pressure in patients with mild to moderate hypertension. Nevertheless, only losartan was correlated with a reduction in serum uric acid levels [36].
Conclusion
Valsartan affects the RAAS by competing with Angiotensin II to bind at the AT1 receptor. Studies have shown valsartan alone and combined with other drugs from different classes reduced hypertension and improved mortality and morbidity in patients. Valsartan is rapidly absorbed after oral administration. Valsartan is highly bound to albumin, which limits its distribution to the extravascular compartment. Liver metabolism is not the major elimination route of valsartan, whereby biliary excretion is the major elimination route. Thus, valsartan is not suggested for patients with hepatic dysfunction and biliary cirrhosis. The major advantages of valsartan include a decrease in blood pressure and reduced cardiovascular morbidity and mortality. Valsartan is contraindicated in patients with underlying hypovolemic problems. Valsartan’s peak plasma concentration is high after the administration of the fast-dissolving formulation and possessed a higher risk of symptomatic hypotension compared to the usual oral tablet formulation. There is a lack of investigation conducted to evaluate the usage of valsartan in pregnancy and breastfeeding. No clinically notable pharmacokinetic interactions were indicated when valsartan was coadministered with other notable therapeutic drugs. Losartan and valsartan are comparably efficient in decreasing blood pressure in patients with mild to moderate hypertension. Nevertheless, only losartan therapy was correlated with reduced serum uric acid levels.
References
https://dailymed.nlm.nih.gov/dailymed/lookup.cfm?setid=8478a05f-38fd-412d-af49-69a73a787c8f [October 2019].
