ASSESSMENT OF CYTOTOXIC ACTIVITY TOWARDS PC3 CELL LINE OF PEPTIDE ESTERS OF GALANTAMINE: GAL-LEU AND GAL-VAL
Dobrina Tsvetkova1*, Lyubomir Vezenkov2, Tchavdar Ivanov2, Dancho Danalev3, Ivanka Kostadinova4
|
|
|
ABSTRACT
The current work's objective was to examine the cytotoxic activity of recently created peptide esters of Galantamine: GAL-LEU and GAL-VAL on prostate cancer PC3 cell lines. For the estimation of the cytotoxic effect of Galantamine derivatives, the MTT reduction assay was applied. PC3 cells were triplicate exposed separately to each of the peptide esters, applied in different concentrations (1.875 mМ ÷ 30 mМ). In the MTT test, the reduction of tetrazolium salt MTT resulted in the creation of formazan, whose absorbance was measured spectrophotometrically at wavelength 570 nm. The experimental results show that peptide ester GAL-LEU at 30 mM inhibits 55.36% of PC3 cell growth with an index of cell viability of 44.64 %. The lower antiproliferative effect of derivative GAL-VAL was proven by the fact that 30 mM inhibits 43.96 % of cell growth. The results showed that both of the tested esters had cytotoxic action against the PC3 cell line, but that GAL-LEU has a stronger antiproliferative impact than GAL-VAL (IC50 >30 mM), because of its lower value of IC50 = 30.8 mM.
Keywords: Peptide esters, Galantamine, MTT, PC3 cell line
Introduction
Cancer is a disease involving unregulated cell growth. An important risk factor for cancer is old age [1]. Other factors are smoking [2], environmental agents such as exposure to chemicals, alcohol, drugs, sunlight, ionizing radiation, electromagnetic fields [3], and infectious agents [4]. Cytotoxic chemotherapy is applied for different types of cancer including lung, pancreatic and colorectal cancer [5].
The most used anticancer drugs are antimetabolites [6], cytotoxic antibiotics anthracyclines [7], and inhibitors of topoisomerase [8]. Flutamide, Bicalutamide, and Nilutamide are applied for prostate cancer [9]. It was discovered that extracts from different plants like species of Astragalus possess anticancer activity [10].
Alzheimer's disease is treated using Galantamine [11] improves cognitive functions by the following mechanisms [12]: inhibition of acetylcholinesterase [13]; stimulation of α7-subtype binding position of nicotinic acetylcholine receptors [14] and antioxidant activity [15, 16].
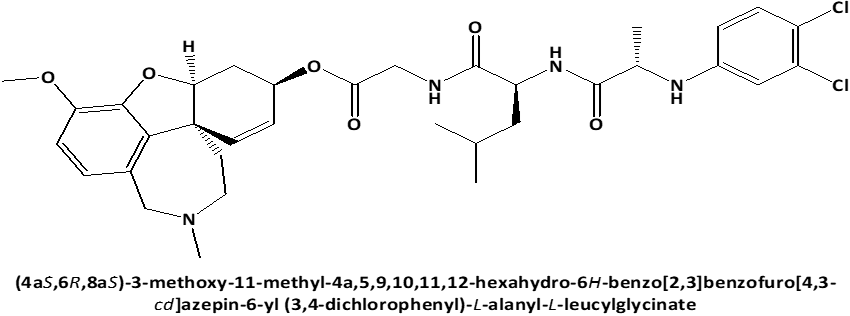
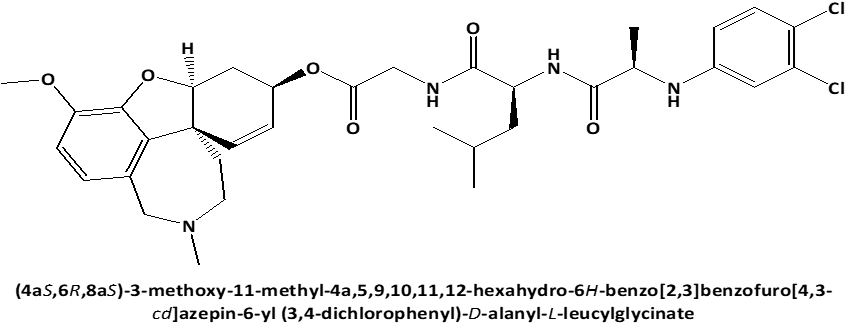
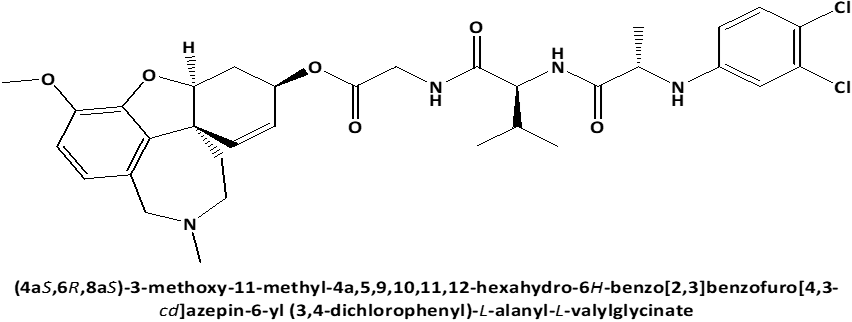
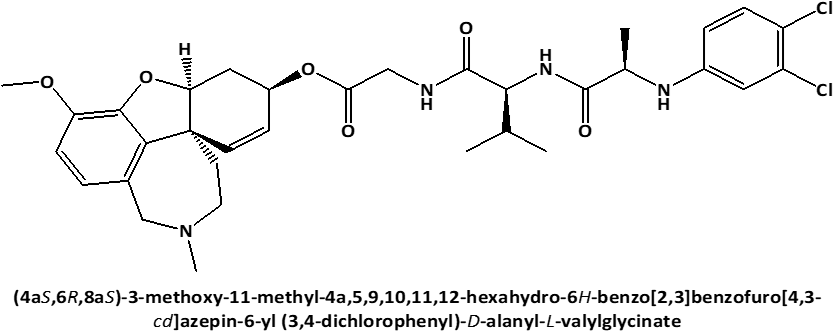
In connection with that L-Leucyl-L-Leucine methyl ester induces apoptosis on cell lines [17] and because the discovery of cytotoxic properties of new compounds can increase the possibility of obtaining the new in-vivo effective pharmaceutical agents for anticancer therapy, the current work's objective was to examine the cytotoxic activity against on PC3 prostate cancer cells [18, 19] of newly synthesized peptide esters: 6-O-N-[N-(3.4-dichlorophenyl)-D, L-Alanyl]-L-Leucyl-Glycil-Galantamine (GAL-LEU) and 6-O-N-[N-(3.4-dichlorophenyl)-D, L-Alanyl]-L-Valil-Glycil-Galantamine (GAL-VAL) [20], for which has been observed to exert both inhibitory activities against acetylcholinesterase and g-secretase [21] and which exhibit antioxidant qualities in the FRAP (ferric reducing/antioxidant power) process [22, 23].
Materials and Methods
 |
|
a) |
 |
|
b) |
 |
|
c) |
 |
|
d) |
|
Figure 1. Chemical structures of GAL-LEU and GAL-VAL. |
Standard MTT (3-[4.5-dimethylthiazole-2-yl]-2.5-diphenyl-tetrazolium bromide), dimethylsulfoxide, fetal bovine serum (FBS), 100 IU/ml Penicillin, 100 µg/ml Streptomycin, 0.25 % Trypsin EDTA 1X.
An accurately weighed quantities of the examined peptide esters GAL-LEU and GAL-VAL were dissolved separately in dimethylsulfoxide to obtain concentrations: 1.875 µM; 3.75 µM; 7.5 µM; 15 µM; 30 µM.
An accurately weighed quantity of MTT was dissolved in phosphate buffer solution to obtain a solution with a concentration of 5 mg/ml. This solution is stable for 1 month at storage at 4 °С.
Dulbecco's Modified Eagle Medium was used for the cultivation of the PC3 prostate cancer cell line for the assessment of the cytotoxic activity of peptide esters.
To examine the PC3 cell line's susceptibility to the cytotoxicity of Galantamine peptide esters, the MTT test of Mosmann was applied [18]. Into each well separately were added 200 µl of solution of respective ester in fresh medium in different dilutions (1.875 µM ¸ 30 µM). For each of the examined esters in each concentration experiments were performed in triplicates. The average values were calculated. After 48 hours of exposure on esters 200 µl 0.5 mg/ml MTT were added directly to each well. The plates were then incubated for a further 4 hours at 37°C with 5% CO2. To solubilize the produced formazan, 100 µl of dimethylsulfoxide was added to each well after the supernatant was removed. The formazan's absorbance was measured at λ = 570 nm.
96 Microplates with flat bottoms were used. By using the established MTT colorimetric test, the compounds' cytotoxic activity was assessed. PC3 cells were added to Dulbecco's Modified Eagle Medium in 75 cm2 flasks. After addition to a medium of 5 % fetal bovine serum, 100 µg/ml Streptomycin, and 100 IU/ml Penicillin, PC3 cells were incubated in a fully humidified atmosphere of 5% CO2 at 37 °C for 24 h. In the obtained phase of exponential growth, the cells were trypsinized, ad centrifuged. Haemocytometer was used for the determination of the content of cells. Cell culture with a concentration of 1.105 cells/ml was obtained by diluting with a particular volume of medium. 100 µl/well was added to 96-well plates. Samples were incubated for 24 hours at 37 °C in a thoroughly humidified 5% CO2 environment. The culture media was withdrawn after incubation.
Results and Discussion
The cytotoxic activity of peptide esters GAL-LEU and GAL-VAL against PC3 prostate cells was estimated by using the standard MTT colorimetric test.
As (A(+)) control PC3 line was treated with MTT without peptide esters. As (A(-)) control PC3 cell line was dissolved in a culture medium without MTTand peptide esters. For the investigation of the antiproliferative effect of peptide esters, the PC3 cell line was treated separately and duplicated with GAL-LEU in different concentrations (1.875 mМ ÷ 30 mМ) and triplicated with GAL-VAL in 30 mМ. MTT assay was applied. As a standard was used Doxorubicin. In Таble 1. absorbances of positive A (+) and negative A (–) controls and of formazan obtained after treatment of PC3 cell line with tested compounds are summarized.
Table 1. Absorbances of controls and of formazan produced from the GAL-LEU- and GAL-VAL-treated PC3 cell line.
|
Cell line |
PC3 |
|||||||
|
N: |
GAL-LEU |
GAL-VAL |
||||||
|
1. |
0.947 |
0.065 |
1.155 |
0.064 |
||||
|
2. |
0.909 |
0.063 |
1.007 |
0.063 |
||||
|
3. |
0.883 |
0.061 |
1.019 |
0.059 |
||||
|
4. |
0.930 |
0.063 |
1.021 |
0.065 |
||||
|
5. |
0.930 |
|
1.053 |
|
||||
|
6. |
|
|
1.077 |
|
||||
|
X |
0.920 |
0.063 |
1.055 |
0.063 |
||||
|
SD |
0.025 |
0.002 |
0.055 |
0.003 |
||||
|
CGAL-LEU [mM] |
Absorbances of formazan [AU] |
|||||||
|
|
1 |
2 |
3 |
X |
SD |
|||
|
3.75 |
0.816 |
0.830 |
|
0.823 |
0.010 |
|||
|
7.5 |
0.864 |
0.802 |
|
0.833 |
0.044 |
|||
|
15 |
0.751 |
0.837 |
|
0.794 |
0.061 |
|||
|
30 |
0.465 |
0.426 |
|
0.446 |
0.028 |
|||
|
CGAL-VAL [mM] |
Absorbances of formazan [AU] |
|||||||
|
30 |
0.666 |
0.615 |
0.576 |
0.619 |
0.045 |
|||
In Figure 2 is illustrated that the increase of concentration correlates with the decrease of formazan’s absorbance and of cell viability.
|
|
|
Figure 2. Absorbance – concentration relation for GAL-LEU. |
Experiments were applied in duplicate.
Sample 1 = 1st experiment
Sample 2 = 2nd experiment
In Table 2. the data for the activity of esters on inhibition of cell growth (%) are summarized.
Table 2. Effect of GAL-LEU and GAL-VAL on inhibition of PC3 cell growth.
|
CGAL-LEU [mM] |
Inhibition PC3 cell growth [%] |
||||
|
1 |
2 |
3 |
X |
SD |
|
|
3,75 |
12.11 |
10.48 |
|
11.30 |
1.15 |
|
7,5 |
6.51 |
13.75 |
|
10.13 |
5.12 |
|
15 |
19.70 |
9.66 |
|
14.68 |
7.10 |
|
30 |
53.08 |
57.63 |
|
55.36 |
3.22 |
|
CGAL-VAL [mM] |
Inhibition PC3 cell growth [%] |
||||
|
1 |
2 |
3 |
X |
SD |
|
|
30 |
39.22 |
44.36 |
48.29 |
43.96 |
4.55 |
In Figure 3 the cytotoxic effect of GAL-LEU against the PC3 cell line is illustrated.
|
|
|
Figure 3. Cytotoxic effect of GAL-LEU against PC3 cell line. |
Experiments were applied in duplicate.
Sample 1 = 1st experiment
Sample 2 = 2nd experiment
The obtained data for the inhibition concentration IC50 are GAL-LEU: 30.8 mM; GAL-VAL: > 30 mM.
In Table 3. the data for activity of esters on the index of cell viability V (%) and are presented.
Table 3. Effect of GAL-LEU and GAL-VAL on proliferation of PC3 cell line.
|
CGAL-LEU [mM] |
Index of cell viability of PC3 cell line [%] |
||||
|
1 |
2 |
3 |
X |
SD |
|
|
3.75 |
87.89 |
89.52 |
|
88.70 |
1.15 |
|
7.5 |
93.49 |
86.25 |
|
89.87 |
5.12 |
|
15 |
80.30 |
90.34 |
|
85.32 |
7.10 |
|
30 |
46.92 |
42.37 |
|
44.64 |
3.22 |
|
IC50 |
29.84 |
31.76 |
|
30.80 |
1.38 |
|
CGAL-VAL [mM] |
Index of cell viability of PC3 cell line [%] |
||||
|
1 |
2 |
3 |
X |
SD |
|
|
30 |
60.78 |
55.64 |
51.71 |
56.04 |
4.55 |
In Figure 4 the accordance between the concentration of GAL-LEU and the index of cell viability V (%) is demonstrated.
|
|
|
Figure 4. Effect of GAL-LEU on PC3 cell viability. |
Experiments were applied in duplicate.
Sample 1 = 1st experiment
Sample 2 = 2nd experiment
Prostate adenocarcinoma is considered to be the second most commonly diagnosed malignancy [24] and the second highest factor of cancer-related death in men [25]. Prostate cancer cell proliferation is regulated by androgens via the androgen receptor [26]. Aggressive prostate cancer is associated with up-regulation of caveolin-1 [27]. The most often applied therapy for patients with metastatic prostate cancer is the combination of Docetaxel and Prednisone [28]. Other treatments include surgery, radiation, and hormonal therapy, naturally occurring compounds: lycopene, curcumin [25], genistein [29], and natural polyphenol Gallic acid [30]. Liposomal hydroxy aluminum phthalocyanine gel has the potential for effective photodynamic therapy of prostate carcinoma [31]. Analogs of vitamin D can be utilized to treat and prevent prostate cancer since vitamin D deficiency has been associated with increased risk of illness [32, 33].
The PC3, LNCaP, and DU145 cells [34] are being widely used to analyze and characterize the development of human prostate cancer in vitro and in vivo [35]. An epithelial prostatic adenocarcinoma cell line called PC3 is utilized to study the biochemical alterations in advanced prostate cancer cells and gauge how well they respond to chemotherapy drugs. The PC3 cell line was created in 1979 [36].
For the investigation of cell viability and proliferation [37] widely is applied the MTT test of Moosmann, in which soluble yellow MTT is reduced by the mitochondrial enzyme activity of viable cells into soluble in dimethylsulfoxide violet formazan, which absorbance can be measured spectrophotometrically at λ = 570 nm [18]. The index of cell viability V (%) and cell growth inhibition I (%) were calculated. The amount of activity is a gauge of the viability of the cells since the decrease of MTT can only occur in metabolically active cells. A decrease in the rate of cell growth is shown by absorbance values that are lower than those of the control cells. An increase in cell proliferation is indicated by a greater absorbance rate.
It has been demonstrated that indole-3-carbinol inhibits the growth of PC3 and DU145 cells leading to apoptosis [38]. Pinocembrin (5, 7-dihydroxy flavanone) shows a potent antiproliferative effect against PC3, DU-145 and LNCaP cells [39]. Propolis extracts suppresses the viability of human prostate cancer cells [40].
According to studies, arachidonic acid and its metabolites, which are created by the enzyme 5-lipoxygenase, promote the proliferation of prostate cancer cells. The natural products that act as 5-lipoxygenase inhibitors include caffeic acid [41], curcumin [42], quercetin [43], luteolin [44], resveratrol [45], rosmarinic acid [46], nordihydroguaiaretic acid [47].
It has been observed that Caffeic acid phenethyl ester dosage dependently inhibits of LNCaP, DU-145, and PC3 cells [48]. LNCaP and DU 145 cell-growth is suppressed by Curcumin from Curcuma longa L. and genistein, daidzein, and glycitein. Isoflavones alone do not have as strong an inhibitory effect on cell growth as does the combination with Curcumin [49].
Curcumin suppresses proteins Bcl-2 and Bcl-x what inhibit apoptosis. Curcumin activates pro-apoptotic proteins from the Bcl-2 family and caspases [50]. According to research, luteolin inhibits the growth of human prostate tumors [43]. Quercetin is known to cause death of prostate cancer cells, which is a result of stimulation of caspases [44]. Nordihydroguaiaretic acid increases Ca2+ concentration by releasing of Ca2+ from the endoplasmic reticulum [47].
Tumors can be a result of stem cells mutations [51]. MTT test of Moosmann is used for the estimation of effect of plant extract against cancers. Some tumors such as lung, breast, prostate, and cervical are high spread in population, while others like urinary bladder primary squamous cell carcinoma are with rare cases [52]. In Saudi Arabia are spread cervical cancer, thalassemia and sickle-cell anaemia [53].
It has been reported that for the estimation of the citotoxic activity of extracts of Rheum ribes L. towards cell lines A-549 and KB [16], Acalypha wilkesiana against cervical cancer HeLa cell line [19] and MCF-7 breast cancer cell line [23] and ginger on cell viability in breast and pancreatic cancer [54], was used MTT test. For these assay a specific cell-line-grow mediun was applied [55].
In our previous experiments, the survival of 3T3 cells was assessed using Moosmann's MTT assay. In concentration 30 μM GAL-VAL inhibits 88.32 % of 3T3 cell growth and exerts cytotoxic activity with IC50 = 23.17 μM [56]. GAL-LEU in concentration 30 μM suppresses 99.9 % of 3T3 cells and possesses antiproliferative cytotoxic activity with IC50 = 19 μM [57].
The current investigation of the Galantamine peptide esters GAL-LEU and GAL-VAL [58] effects on the inhibition of cell growth PC3 cell line that was treated triplicated separately with each of the examined peptide esters in different concentrations (1.875 mМ ¸ 30 mМ). In the applied MTT test of Mosmann, the accordance of formazan is proportional to viability cell lines.
The cytotoxic effect was estimated as concentration that suppresses 50 % of PC3 cells (IC50). Cell growth inhibition I (%) and index of cell viability V (%) by the following equations were calculated:
|
|
(1) |
|
|
(2) |
V (%) – index of cell viability
I (%) – cell growth inhibition
At – mean absorbance value of formazan, obtained after treatment of the examined cells with test compounds
A(+) – mean absorbance of formazan, obtained with the positive control (treated with an MTT solution without the test chemicals in the studied cell line
A(-) – mean absorbance value of formazan, obtained with negative control (MTT, without the examined chemicals.
The experimental results show that GAL-LEU at concentration 30 mM inhibits 55.36 % of PC3 cell growth with an index of cell viability of 44.64 %. GAL-VAL in concentration 30 mM inhibits 43.96 % of cell growth with an index of cell survival of 56.04 %. These results prove the lower antiproliferative effect of GAL-VAL in comparison with GAL-LEU.
Conclusion
The treatment of the PC3 cell line with peptide esters decreased formazan concentration and absorbance, indicating their ability to suppress growth. The outcomes of the experiment demonstrated that GAL-LEU slowed the growth of PC3 cancer cells. The experimental findings demonstrated that both esters had cytotoxic effects on the PC3 cell line, but that GAL-LEU has a stronger cytotoxic impact than GAL-VAL (IC50 > 30 mM) because of its lower IC50 of 30.8 mM.
The antiproliferative activity of peptide esters is lower compared to standard Doxorubicin activity (IC50 = 1.698 mM ± 0.285 mM).
Acknowledgments: The authors are gratefully acknowledged Rizwana Malik, Sadia Siddiq, and Prof. Iqbal Choudhary from ”Dr. Panjwani Center for Molecular Medicine and Drug Research, ICCBS, University of Karachi, Pakistan, for skillful technical assistance, great experimental support, extending laboratory competence and for their continued scientific correspondence, cooperation, and help. The authors gratefully acknowledged Prof. Danka Obreshkova for scientific consultation and cooperation.
Conflict of interest: None
Financial support: None
Ethics statement: None
