Role of IL-10 In Inhibition of Trichomonas Vaginalis Killing And IFN- Γ Activated Macrophages
Noor Ibrahim Abdulzahra*, Jasim Hameed. Taher, Raja Jawad Mohamed
|
|
Medical laboratory Techniques Department, Kufa Technical Institute, Al-Furat Al-Awsat Technical University, 31003 Al-Kufa, Iraq. |
ABSTRACT
This study was carried out to evaluate the cytokines profile, Interleukin-10 (IL-10), and Interferon-gamma (IFN-γ) in the serum of infected women using ELISA Techniques. The blood samples were collected from 64 women infected with Trichomonas vaginalis and suffering from vaginal discharge attending the outpatient clinics at Al-Zahra'a children and Maternity Hospital and private clinics, Najaf, between June 2016 to March 2017, in addition to 32 healthy patients as a control. Blood sera were obtained from those women. The cytokines IL-10 and IFN-γ were evaluated in serum. There was a significant increase in both cytokines in the infected women, and also both cytokines showed significant differences. These trichomoniasis patients showed higher production of IL-10 and lower IFN-γ, resulting in a higher ratio of IL-10: IFN-γ compared to the control group ratio.
Keywords: T.vaginalis, IL-10, IFN-γ, IL-10: IFN –γ ratio
Introduction
T. vaginalis infection occurs worldwide [1-3] and exists as flagellates and ameboid trophozoites and the pseudocystic stages [4, 5]. The trophozoites reside on the mucosal surface of the vagina in infected women [6]. The parasite lives in the female lower reproductive tract, rarely the male urethra, and is classified as a sexually transmitted agent [7]. Cytokines produced by TH1 cells enhance protective immunity against some trial protozoa and characterized by increased IFN-γ and increases expression of IL-10 which down-regulates TH1 cells [8]. The role of cytokines as well as the link between humoral and cellular immune responses in human trichomoniasis determine the production of IL-10 and IFN-γ
and increases expression of IL-10 which down-regulates TH1 cells [8]. The role of cytokines as well as the link between humoral and cellular immune responses in human trichomoniasis determine the production of IL-10 and IFN-γ [9]. Some biochemical and immunological aspect of T. vaginalis was studied in Iraqi women [10]. A cell-mediated immune response is evoked [11]. T-cell subsets and cytokines serve a central function as key factors in the regulation of mucosal response in various parasitic infections [12]. There was less information on the cell-mediated immune response during human trichomoniasis to the cytokines profile IL-10 and IFN-γ
[9]. Some biochemical and immunological aspect of T. vaginalis was studied in Iraqi women [10]. A cell-mediated immune response is evoked [11]. T-cell subsets and cytokines serve a central function as key factors in the regulation of mucosal response in various parasitic infections [12]. There was less information on the cell-mediated immune response during human trichomoniasis to the cytokines profile IL-10 and IFN-γ that represent the T-helper, so the aim of this study is to assess in vitro, lymphocytes functions by cytokine productions to further assess the involvement of TH1 and TH2 cytokines in the regulation of the immune response.
that represent the T-helper, so the aim of this study is to assess in vitro, lymphocytes functions by cytokine productions to further assess the involvement of TH1 and TH2 cytokines in the regulation of the immune response.
Materials and Methods
Sixty-four female patients attending the Obstetrics and Gynecology outpatient department of Al-Zahra'a Children and Maternity Hospital and private clinics, Najaf were collected between September 2016 to March 2017. All patients were non-pregnant, aged between 25-50 years. All of them complained of vaginal discharge, menopausal women who were receiving hormonal replacement therapy, women with genital prolapses, women with overt genital bleeding or ovarian tumors, those receiving antibiotics therapy, and recent use of the vaginal product were excluded from this study. Sixty-four healthy-looking, age-matched women, with no vaginal or other systemic infections were selected as control. Some information was taken for each patient such as name, gender, age, address, etc. All blood samples and other subjects were collected on Iraqi Health Ministry's permission for investigated patients.
Specimens Collection
Blood
Three ml of venous blood was collected from each woman after disinfecting the anti-cubital fossa with 70% ethanol [13]. Vein puncture was carried out with a 5 ml disposable syringe with a 23-gauge needle, after applying a tourniquet. Three ml were placed in plain tubes and serum was collected by allowing the blood to clot for one hour at room temperature. The clot was then detached from the walls of the tube, then centrifuged (Hettich) for 10 minutes at 4°C at 450xg. The serum was aspirated and dispensed into sterile glass tubes (0.1 ml in each) and kept at 20°C until used for the determination of cytokines concentration.
Vaginal Swab
Vaginal swabs were collected from symptomatic patients presenting with vaginal discharge, itching, and dysuria [11]. The patients were placed in a lithotomic position, and then through a non-lubricated sterile bivalve speculum, swabs were taken from the posterior fornix by a sterile cotton-tipped applicator [14]. The swab was placed in a tube containing 0.5 ml physiological saline solution (0.9% NaCl) for mount examination. The tube was carried immediately to the laboratory, then gently shook and a slide was prepared for examination immediately under the light microscope (Olympus) at 10x and 40x objectives to detect the motile organism [15].
Determination of Cytokines
Interferon-γ  and Interleukin-10
and Interleukin-10
Serum levels of IFN-γ and IL-10 were measured by means of the enzymatic immunoassay using ELISA kit (Mabtech AB. Sweden) as recommended by the manufacturer. The assays had detected limits of 2pg/ml for IFN-γ
and IL-10 were measured by means of the enzymatic immunoassay using ELISA kit (Mabtech AB. Sweden) as recommended by the manufacturer. The assays had detected limits of 2pg/ml for IFN-γ and 0.5pg/ml for IL-10. The standard curves were done (Fig 1 and 2).
and 0.5pg/ml for IL-10. The standard curves were done (Fig 1 and 2).
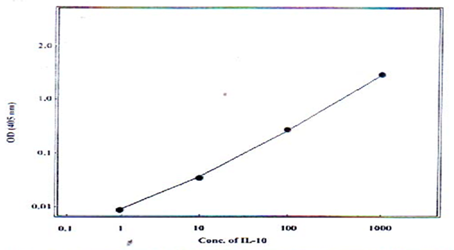
Fig. 1: Standard curve for estimation of IL-10 by ELISA technique.
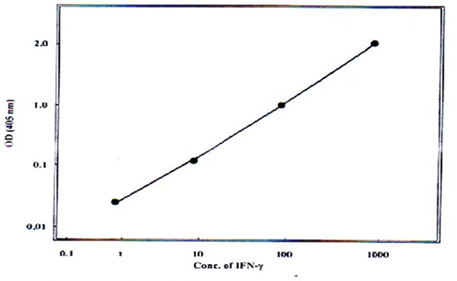
Fig. 2: Standard curve for estimation of IFN-γ by ELISA technique.
Statistical Analysis
The data were analyzed using the available software package. The results were presented as number, percentage, and mean ± SD whenever possible. The data were analyzed by using analysis of variance (ANOVA) test according to the Statistical Analysis System [15].
SD whenever possible. The data were analyzed by using analysis of variance (ANOVA) test according to the Statistical Analysis System [15].
Results
Cytokine levels:
Interferon-gamma (IFN-γ )
)
A significant increase (P<0.05) of serum IFN-γ was recorded in patients with T. vaginalis infection (0.0378∓
was recorded in patients with T. vaginalis infection (0.0378∓ 0.014) in comparison to the control group (0.0056∓
0.014) in comparison to the control group (0.0056∓ 0.00077) (Table 1).
0.00077) (Table 1).
Interleukin-10 (IL-10)
There was an increase of IL-10 levels (P<0.001) in the sera of patients (0. 136∓ 46) in comparison to the control group (0.0177 ± 0.14) (Table 1).
46) in comparison to the control group (0.0177 ± 0.14) (Table 1).
IL-10/IFN-γ ratio
ratio
Trichomoniasis patients exhibited higher production of IL-10 and lower IFN-γ  levels, resulting in a higher ratio of IL-10: IFN-γ
levels, resulting in a higher ratio of IL-10: IFN-γ (3.59) compared to that of control (3.16) (Table 2).
(3.59) compared to that of control (3.16) (Table 2).
Table 1: Cytokines production in the sera of 64 trichomoniasis patients and 32 healthy control.
|
Types of Cytokine |
Patients Mean Conc. ∓ |
Control MeanConc.∓ |
P-value |
|
IFN-γ |
0.0378±0.014 |
0.0056± |
<0.05 |
|
IL-10 (pg/ml) |
0.136± |
0.0177± |
<0.001 |
Table 2: The ratio of IL-10:IFN-γ in the sera of trichomoniasis patients compared to healthy control.
in the sera of trichomoniasis patients compared to healthy control.
|
Group |
Mean IL-10 |
Mean IFN-γ |
Ratio IL-10:IFN-γ |
|
Control |
0.0177 |
0.0056 |
3.16 |
|
Patient |
0.136 |
0.0378 |
3.59 |
Discussion
The cytokines which were evaluated in this study were chosen based on the fact that they represent important numbers of proinflammatory, regulatory T-helper1 (IFN-γ ) and T-helper 2 (IL-10) cytokines. IFN-γ
) and T-helper 2 (IL-10) cytokines. IFN-γ  and IL-10 cytokines have been detected in the patients’ serum. However, data from this study showed a significant increase in the concentration of IL-10 and a significant increase in the concentration of IFN-γ
and IL-10 cytokines have been detected in the patients’ serum. However, data from this study showed a significant increase in the concentration of IL-10 and a significant increase in the concentration of IFN-γ  in serum. Although the level of IFN-γ
in serum. Although the level of IFN-γ is significantly elevated yet still, the value of IL-10 is significantly higher than IFN-γ
is significantly elevated yet still, the value of IL-10 is significantly higher than IFN-γ  (high IL-10:IFN-γ
(high IL-10:IFN-γ ratio). The increase in IL-10 over IFN-γ
ratio). The increase in IL-10 over IFN-γ could be due to the fact that antibody production is predominant in T. vaginalis infection [16] which is achieved via the production of IL-10 [17]. Furthermore, studies indicate that IL-10 is produced by a large variety of cells in addition to the lymphocytes, including B cells and macrophage [18]. IL-10 also inhibits the synthesis of a wide variety of proinflammatory monokines by macrophage effector functions against different pathogens [19]. The main method by which IL-10 inhibits IFN-γ
could be due to the fact that antibody production is predominant in T. vaginalis infection [16] which is achieved via the production of IL-10 [17]. Furthermore, studies indicate that IL-10 is produced by a large variety of cells in addition to the lymphocytes, including B cells and macrophage [18]. IL-10 also inhibits the synthesis of a wide variety of proinflammatory monokines by macrophage effector functions against different pathogens [19]. The main method by which IL-10 inhibits IFN-γ synthesis by NK and TH1 cells is though inhibition of macrophage IL-12 synthesis [20]. It is also noteworthy that the degree of TH1 or TH 2 polarization seems to increase with the chronicity of immune response and can become pathogenic in certain cases [21].
synthesis by NK and TH1 cells is though inhibition of macrophage IL-12 synthesis [20]. It is also noteworthy that the degree of TH1 or TH 2 polarization seems to increase with the chronicity of immune response and can become pathogenic in certain cases [21].
References
