PHARMACOGNOSTIC, PHYTOCHEMICAL AND PHARMACOLOGICAL ASSESSMENT OF MORINDA TINCTORIA AS ANTI-ULCEROGENIC POTENTIAL
Suresh Chandra1*, Rakesh Kr.Meel 2
|
|
|
ABSTRACT
Introduction- The aerial part of M. tinctoria has many therapeutic implications in traditional Indian medicine. M. tinctoria has antibacterial, antifungal, hepatoprotective, antioxidant, anticancer, antidiabetic, wound healing properties. Aim and Objectives- The study aimed to determine the ulcerogenic potential of the hydro-alcoholic extract by pharmacognostic, phytochemical, and pharmacological assessment. Material and Methods- The preparation of M. tinctoria extract was done with hydro-alcoholic using a vacuum rotary evaporator. Acute-oral toxicity (14 days) and sub-acute oral toxicity (28 days) were performed according to the OECD guidelines. The study was carried out by pylorus ligation, swimming-stress, aspirin-induced ulcer models in Wistar rats. Results- The pharmacognostic analysis was performed according to the I.P. and the total ash was 4.73%, acid-insoluble ash was 0.66%, water-soluble ash was 2.16%, foreign organic matter was 1.80%, loss on drying was 10.34%, alcohol soluble extractive was 56.59%, water-soluble extractive was 7.12%, and ether soluble extractive was 13.76%. A preliminary phytochemical test showed the presence of carbohydrates, amino acids, glycosides, flavonoids, and alkaloids in the hydro-alcoholic extract. Acute-oral toxicity (14 days) and sub-acute oral toxicity (28 days) did not show any effect on body weight and organ weight, and it was non-toxic. In the Pylorus ligation-induced gastric ulcer model, 400mg/kg MTE was significant as compared to standard and revealed Ulcer Index of 5.48±0.01, whereas, MTE at a dose of 400mg/kg displayed 4.92±0.02. In Swimming Stress-induced gastric ulcer model, MTE extract at a dose of 400 mg/kg reported Ulcer Index of 0.58±0.00 and a significantly decreased incidence of ulcers. In Aspirin-induced gastric ulcers in rats, taken orally at a dose of 400 mg/kg, MTE reported Ulcer Index of 3.47±0.01 and no hyperplasia and inflammation were observed. Significant lessening in the ulcer index at a dose of 400 mg/kg was observed in comparison to the standard. MTE greatly decreased ATP hydrolysis by goat gastric ATPase with IC50 by 35 μg/mL. As a positive control, Omeprazole (10-50μg / mL) was utilized to decrease the activity of H+-K+ ATPase with IC50= 29 μg/mL. Conclusion- M. tinctoria extract (MTE) effectively prevents the gastric mucosa from the threat of ulcers caused by pylorus ligation, swimming-stress, and aspirin-induced ulcers.
Keywords: Peptic Ulcer, Morinda tinctoria, Acute toxicity study, Sub-acute toxicity study, Pylorus ligation, Swimming stress, Aspirin, H+-K+ ATPase.
Introduction
There are about 300 to 315 thousand of the plant variety in the kingdom Plantae from which many species are used as medicines for ages [1, 2]. Countless studies have examined the function of medicinal plants and their wound healing ability [3-8]. A peptic ulcer is a condition of the digestive tract that is referred to as the corrosive peptic damage that causes the submucosa or muscularis propria to tear through the mucosa [9]. Peptic ulcers can be found in the esophagus as well as in the diverticulum of Mackel but it is in the stomach or the proximal duodenum [10]. Etiological factors such as less eating routine, stress, hypersecretory acid environment, long-haul use of NSAIDs, H.pylori disease, high alcohol and smoking intake, and certain genetic variables are also responsible for this disorder [11]. In the past 30 years, a sharp decrease in hospitalization and mortality rates and incidence ratios was noted [12, 13]. In the general population, peptic ulcer disease's lifetime inevitability has been measured at about 5-10% and an occurrence rate of around 0.1-0.3% per annum [14].
Morinda species are also known as the traditional plants found in the tropical countries used as herbal medicines for years. Morinda tinctoria belongs to the family Rubiaceae is commercially known as Nuna, Aal, and Indian Mulberry, locally known as Togaru, which is a small tree or evergreen shrub around 5-10 m in height. The shape of its leaves is elliptical to lanceolate and is around 15-25 cm in total. The blossoms are white, cylindrical, and scented, and are around 2 cm long. Fruits are green in color and are about 2-2.5 cm in diameter. It is cultivated in southern Asia, the Pacific Islands, and Eastern India and mostly grows like a wild shrub in the agricultural lands. In India, Morinda is cultivated in Kerala and Tamil Nadu whereas, M.tinctoria species is widely found in most parts of the Tamil Nadu regions and fewer parts of Kerala. The therapeutic benefits are been noted by the South Indian associative and thus M.Tinctoria is been traditionally used in the Ayurveda and Siddha systems of Indian Medicines [15-18]. As all parts of the M.tinctoria species are therapeutically efficient, it can be used as astringent, deobstruent, emmenagogues, and in gout pain relief. Early studies recorded that this plant's leaf, root, and fruit extract had antibacterial, pain-relieving, against oxidant, calming, astringent, diuretic, soothing, hypotensive limit (lower pulse) and hostile to ulcer activity [19]. Hence, the aerial part of M.tinctoria is used in treating peptic ulcer disease.
Materials and Methods
1. Plant Collection- The aerial part of M. tinctoria was collected from Madurai, Tamil Nadu and was taxonomically identified and verified by Dr. Naveen K. Ambasht (Head and Associate Professor, Botany Department, Christ Church College, Kanpur), where the voucher specimen was deposited.
1.1 Extraction Procedure of M.tinctoria Aerial Part- The fresh aerial part of M. tinctoria was ordered from Madurai, Tamil Nadu. The aerial part was dried completely in the daylight and then powered for further use. The extraction procedure was carried out by the use of hydro-alcoholic solvent of ethanol and water taken in the ratio of 1:1. A weighed quantity of the powder (1500 g) was taken in a big jar in which the hydro-alcoholic solvent was mixed in an equivalent ratio. The powder was macerated for about 72 hours in the solvent. Then, the soaked powder was filtered by the cotton cloth and the filtrate was vacuum dried using a rotary evaporator, and concentrates were stored at room temperature.
1.2 Pharmacognostic Analysis of powder of M.tinctoria- The aerial part of M.tinctoria was dried and mechanically powdered and further used for pharmacognostic and phytochemical analyses. The analytical procedures mentioned in Indian pharmacopeia were followed for the calculation of ash values (total ash, acid-insoluble ash, water-soluble ash), foreign organic matter, extractive values (alcohol soluble extractive, water-soluble extractive, ether soluble extractive), and loss on drying (LOD) at 110ºC.
1.3 Phytochemical Analysis- The qualitative analysis of the hydro-alcoholic extract of M. tinctoria was done to identify various classes of active constituents such as carbohydrates, proteins, steroids, amino acids, glycosides, flavonoids, alkaloids, tannins, and phenols using standard procedures.
2. Animals- Male Wistar rats (150-200gm) were utilized for acute, sub-acute, and screening models. The room temperature where rats were housed was maintained about 22±3°C, relative humidity of 65%, and 12-h light/dark cycle was managed to maintain with the support of artificial light. The bedding was changed 3 times within 7 days interval. Rats were given a normal diet and water was consumed ad libitum. All study was conducted in compliance with the protocols for the treatment and usage of the rats, which are used in the laboratory and have been approved by CPCSEA guideline (CPCSEA/AC/09/1273).
2.1 Acute Toxicity Study- An acute oral toxicity analysis was conducted by the Organization for Economic Cooperation and Development (OECD) 423 for extracts to evaluate safer doses using an acute oral toxic class procedure. Five male Wistar rats were taken and four were given starting doses of 500 mg/kg, 1000, 2000, and 4000 mg/kg (body weight/p.o) of MTE, and one was kept normal. For three days, rats were monitored to assess significant physiological changes like body weight and other indications of toxicity. Then, the procedure was repeated with the same dosage amount again for 7 more days and the assessment was done on the 14th-day [20].
2.2 Sub-acute Toxicity Study- Repeat oral toxicity studies have been conducted as per OECD Guideline 407. The rats were first divided into five groups containing four (male rats) total. Group Ι was given 10 ml/kg distilled water, (bd.wt.), and was treated as normal. Group ΙΙ received 500 mg/kg, group ΙΙΙ received 1000 mg/kg, group ΙV received 2000 mg/kg and group V received 4000 mg/kg (body weight/ p.o) of MTE accordingly. The drug was delivered every day for 28 days at the exact timings, and morbidity and mortality were reported at least twice daily. Animal body weights were assessed weekly after which animals were sacrificed on the 28th day for the assessment of relative body weight, organ weight, biochemical parameters, and hematological analysis of the animals.
3. Anti-Ulcer Screening Models
3.1 Pylorus ligation induced gastric ulcers in rats
|
GROUPS |
TREATMENT |
|
Group 1 |
Normal control (received saline) |
|
Group 2 |
Standard drug Omeprazole (20mg/kg, bd.wt., p.o) |
|
Group 3 |
Hydro-alcoholic extract of M.tinctoria (200mg/kg, bd.wt., p.o) |
|
Group 4 |
Hydro-alcoholic extract of M.tinctoria (400mg/kg, bd.wt., p.o) |
Male Wistar rats (150-200gm) were kept fasted for a complete day but they were allowed to have water ad libitum before the experiment. Then, they were classified into four groups, each comprising 4 animals. Group I received normal saline, Group II received Omeprazole as a standard (20 mg/kg, bd.wt, p.o.), Group III and Group IV received 200 mg/kg and 400 mg/kg of MTE, (bd.wt, p.o.). 1 hr after the treatment of all groups, animals were anesthetized using anesthetic ether; the abdominal portion was cut with a slight incision in the centerline Pyloric part of the stomach, raised and ligated as described by Shay et al. (1945) [21] preventing friction or disruption of the pylorus blood supply. The stomach was carefully replaced, and interrupted sutures helped in the closure of the abdominal wall. After 4 hours of pyloric ligation, rats were sacrificed by CO₂ euthanasia. The abdomen being cut, and stomach content was poured into the centrifugation tube. The full stomach juice was estimated and centrifuged for over 10 minutes at 2000 rpm. pH, total acidity, as well as free acidity was determined. The area of the ulcer on the surface of the stomach was macroscopically studied and assessed on millimeter-square paper. The amount of area (mm²) was represented as the Ulcer Index (UI). [22-24] The Ulcer index was computed by the given equation [25]:
UI=UN+US+UP×10ˉ¹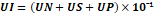
UN= The average no. of ulcers per animal
US= The average severity of scores
UP= % of animals having ulcer {(Total ulcers in a group/Total number of animals in a group) × 100}
3.1.1 pH Determination- 1ml aliquot portion of the gastric juice was dissolved in 1ml distilled water. The solution’s pH was calculated by the use of a pH meter.
3.1.2 Total Acidity Determination- A 1mL gastric juice supernatant was diluted with 1mL of distilled water and chosen to take into a conical flask of 50 ml, few drops of phenolphthalein indicator were added to it and titrated with 0.01N NaOH till a persistent pink color appeared. The consumption amount of 0.01N NaOH was stated. The total acidity was demonstrated as mEq/L by the following equation:
Activity=volume of NaOH×N×100mEqL÷0.1
3.1.3 Free Acidity Determination- Supernatant of gastric juice was titrated with 0.01N sodium hydroxide by introducing an indication of Topfer's reagent before a persistent pink color was detected. The amount of 0.01N sodium hydroxide used was stated, and free acidity was calculated to determine the total acidity using the same equation.
3.2 Swimming Stress-induced gastric ulcers in rats [26]
|
GROUPS |
TREATMENT |
|
Group 1 |
Normal control (received saline) |
|
Group 2 |
Standard drug Famotidine (10mg/kg, bd.wt., p.o) |
|
Group 3 |
Hydro-alcoholic extract of M.tinctoria (200mg/kg, bd.wt., p.o) |
|
Group 4 |
Hydro-alcoholic extract of M.tinctoria (400mg/kg, bd.wt., p.o) |
The rats were kept fasted for a complete day but they were allowed to have water ad libitum before the experiment. Then, they were classified into four groups, each comprising 4 animals. Group I received normal saline, Group II received Famotidine as a standard (10 mg/kg, bd.wt, p.o.) Group III and Group IV received 200 mg/kg and 400 mg/kg of MTE, (bd.wt, p.o.). 30 minutes of post-drug treatment, the rats were made to swim within a circular container (height of 45cm and diameter of 30cm) of water up to about 30cm height. The water temperature was set at 28-29°C. The rats were sacrificed after 3 hours by providing an overdose of anesthetic ether. The stomach was pulled out from the body, removed, and opened. The ulcer areas on the surface of the stomach were macroscopically studied and assessed on millimeter-square paper. The amount of area (mm²) was represented as the Ulcer Index (UI). The Ulcer index was computed by the given equation [25]:
UI=UN+US+UP×10ˉ¹
UN= The average no. of ulcers per animal
US= The average severity of scores
UP= % of animals having ulcer {(Total ulcers in a group/Total number of animals in a group) × 100}
3.3 Aspirin-induced gastric ulcers in rats-
|
GROUPS |
TREATMENT |
|
Group 1 |
Normal control (Positive control) received saline |
|
Group 2 |
Disease control (Negative control)( fasted for 24 hrs+ received aspirin 200mg/kg, bd.wt., p.o) on the 7th day of the experiment. |
|
Group 3 |
Standard drug Omeprazole (20mg/kg, bd.wt., p.o) for 7 days + received aspirin (200mg/kg, bd.wt., p.o) on the 7th day of the experiment. |
|
Group 4 |
Hydro-alcoholic extract of M.tinctoria (200mg/kg, bd.wt., p.o) for 7 days + received aspirin (200mg/kg, bd.wt., p.o) on the 7th day of the experiment. |
|
Group 5 |
Hydro-alcoholic extract of M.tinctoria (400mg/kg, bd.wt., p.o) for 7 days + received aspirin (200mg/kg, bd.wt., p.o) on the 7th day of the experiment. |
Male Wistar rats (120-130gm) were kept fasted for a complete day but they were allowed to have water ad libitum before the experiment. Then, they were classified into five groups, each comprising 4 animals. Group I and Group II received normal saline, Group III received Omeprazole as a standard (20 mg/kg, bd.wt, p.o.), Group IV and Group V did receive 200 mg/kg and 400 mg/kg of MTE, bd.wt, p.o., for 7 days. On the 7th day, 30 min of post-drug treatment, aspirin (200mg/kg, bd.wt, p.o.) was given through oral route to every rat other than normal control (positive control) group with prior fasting of 24 hours. After four hrs, rats were slaughtered by a hard dosage of ether. The stomach was taken down along the greater curvature and managed to open [27]. The mucosa was investigated for complete count and seriousness of ulcers in every stomach. The Ulcer index was computed by the given equation [25]:
UI=UN+US+UP×10ˉ¹
UN= The average no. of ulcers per animal
US= The average severity of scores
UP= % of animals having ulcer {(Total ulcers in a group/Total number of animals in a group) × 100}
3.4 H+-K+ ATPase Inhibition Activity-
Results
1.2 Pharmacognostic Screening-
Table 1- Quantitative Ash values, foreign organic matter, Extractive values, Loss on drying of Morinda tinctoria
|
S. No. |
PHARMACOGNOSTIC ESTIMATIONS |
RESULT |
|
1 |
ASH VALUES Total ash acid-insoluble ash water-soluble ash |
4.73% 0.66% 2.16% |
|
2 |
FOREIGN ORGANIC MATTER |
1.80% |
|
3 |
EXTRACTIVE VALUES Alcohol soluble extractive Water-soluble extractive Ether soluble extractive |
56.59% 7.12% 13.76% |
|
4 |
LOSS ON DRYING |
10.34% |
1.3 Phytochemical Analysis-
Table 2- Qualitative analysis of Morinda tinctoria in aerial part (presence, absence)
|
S. NO. |
PHYTOCHEMICALS |
RESULT |
|
|
1.
|
|
Tests for Carbohydrates Molish's test (General test) For Reducing Sugars: Fehling's test Benedict's test Tests for Monosaccharides: Barfoed's test Tests for Non-Reducing Sugars: Tannic acid test for starch |
Positive Positive
Positive Positive
Negative |
|
2. |
i. ii. iii. iv. v. |
Tests for Proteins: Biuret test (General test) Million's test (for proteins) Xanthoprotein test (For protein-containing tyrosine or tryptophan) Test for protein-containing sulfur Precipitation test- Absolute alcohol 5% HgCl2 solution 5% CuSO4 solution 5% lead acetate 5% ammonium sulfate |
Negative Negative Negative
Positive
Negative Negative Negative Positive Negative |
|
3. |
|
Tests for Steroids: Salkowski Reaction |
Negative |
|
4. |
A. B. |
Tests for Amino Acids: Ninhydrin test (General test) Test for Tyrosine |
Positive Positive |
|
5. |
A. i. ii. B. i. |
Tests for Glycosides: Tests for Cardiac Glycosides Legal's test (For Cardenolides) Test for deoxysugars (Kellar Killani test) Tests for Saponin Glycosides Foam test |
Positive Positive
Negative |
|
6. |
A. B. |
Tests for Flavonoids: Shinoda test Ferric chloride test |
Positive Positive |
|
7. |
A. B. C. D. |
Tests for Alkaloids: Dragendroff's test Mayer's test Hager's test Wagner's test |
Positive Negative Positive Positive |
|
8. |
A. B. C. D. E. F. G. H. |
Tests for Tannins and Phenolic Compounds: 5% FeCl3 solution Lead acetate solution Gelatin Solution Bromine Water Acetic Acid Solution Potassium Dichromate Dilute Iodine Solution Dilute HNO₃ |
Negative Positive Negative Negative Negative Negative Negative Negative |
2. Toxicity Studies-
2.1 Acute Toxicity Study- 5 male Wistar rats had been selected and four were given a starting dose of 500 mg/kg, 1000 mg/kg, 2000 mg/kg, 4000 mg/kg bd wt/p.o. of MTE and one was kept normal. No significant change in body weight was found pre- and post-diagnosis, and no evidence of toxicity. When the procedure was repeated with the same dosage again for 7 more days, no differences were found in the usual behavioral patterns, as well as no clinical symptoms concerning toxicity or fatalities were noted within 14 days.
2.2 Sub-acute Toxicity Study-
2.2.1 Effect on Body Weight-
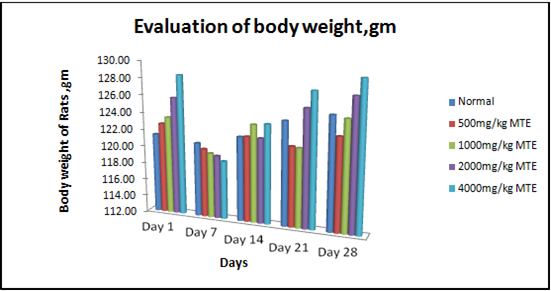
Figure 1- Results were expressed as mean body weight SEM (n = 4) of rats after 28 days of treatment with Hydro-alcoholic extract of Morinda tinctoria. MTE treated groups showed significant changes as compared with control rats (P≤0.05).
2.2.2 Effect on relative Organ Weight-
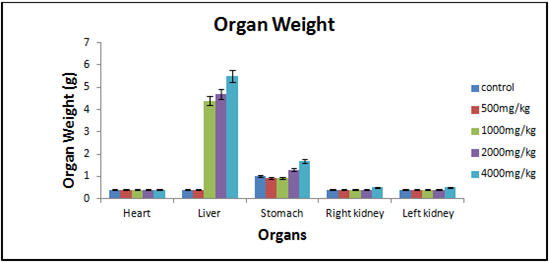
Figure 2- Results were expressed as mean organ weight SEM (n = 4) of rats after 28 days of treatment with Hydro-alcoholic extract of Morinda tinctoria. MTE treated groups showed significant changes as compared with control rats (P≤0.05).
2.2.3 Influence on Biochemical Serum Parameters-
Table 3- The effect of the Aerial part of Morinda tinctoria Hydro-alcoholic extract on Biochemical parameters in rats (values represented as Mean ± SEM).
|
PARAMETERS |
CONTROL |
500mg/kg MTE |
1000mg/kg MTE |
2000mg/kg MTE |
4000mg/kg MTE |
|
Total Cholesterol (mg/dl) |
124.98±0.03* |
102.52 ±0.02* |
255.39 ±0.03* |
92.14 ±0.02* |
69.21 ±0.01* |
|
HDL Cholesterol (mg/dl) |
81.33 ±0.01* |
78.79 ±0.01* |
139.76 ±0.01* |
73.57±0.01* |
62.32 ±0.01* |
|
LDL Cholesterol (mg/dl) |
66.98 ±0.00* |
55.67 ±0.01* |
45.56± 0.01* |
23.66 ±0.01* |
18.73 ±0.00* |
|
Glucose (g/l) (pre-treatment) |
0.72 ±0.09* |
0.95 ±0.05* |
0.79±0.05* |
0.86 ±0.05* |
0.98±0.05* |
|
Glucose (g/l) (post-treatment) |
0.85 ±0.03* |
0.69 ±0.05* |
0.97 ±0.05* |
0.82 ±0.04* |
0.79 ±0.36* |
|
Total Proteins (g/dl) |
6.8±1.2* |
6.4±1.5* |
6.9±1.3* |
7.8 ±1.8* |
7.3±1.9* |
|
Values with superscript (*) for each parameter are significantly the same, P≤ 0.01 and analyzed by one-way ANOVA followed by the Tukey test. MTE- Morinda tinctoria extract |
|||||
** - Non-Significant, * - Significant
2.2.4 Influence on Haematological Parameters-
Table 4- The effect of the Aerial part of Morinda tinctoria Hydro-alcoholic extract on hematological parameters in rats (values represented as Mean ± SEM).
|
PARAMETERS |
CONTROL |
500mg/kg MTE |
1000mg/kg MTE |
2000mg/kg MTE |
4000mg/kg MTE |
|
Haemoglobin (g/dl) |
10.3 ± 0.91* |
10.5 ± 0.89* |
11.28 ± 0.83* |
11.7 ± 0.87* |
12.57 ± 0.94* |
|
WBC (×103/μl) |
7.31 ± 0.48* |
8.13 ± 0.26* |
8.29 ± 0.29* |
8.9 ± 0.32* |
7.97 ± 0.29* |
|
RBC (×106/μl) |
9.46 ± 0.41* |
8.59 ± 0.47* |
9.6 ± 0.57* |
9.69 ± 0.62* |
10.49 ± 0.67* |
|
Platelet( ×103/μl) |
970 ± 0.23* |
973 ± 0.17* |
981 ± 0.26* |
987 ± 0.19* |
998 ± 0.23* |
|
Values with superscript (**) for each parameter are significantly the same, P≤ 0.01 and analyzed by one-way ANOVA followed by the Tukey test. MTE- Morinda tinctoria extract |
|||||
** - Non-Significant, * - Significant
3.1 Pylorus ligation induced gastric ulcers in rats-
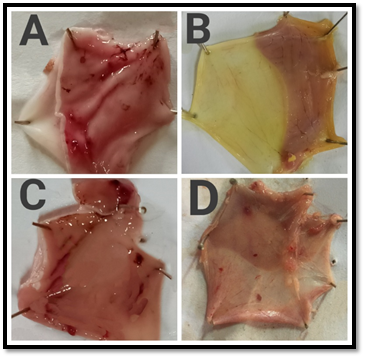
Figure 3- Macroscopic appearance of the gastric mucosa in Pylorus ligation model- A. Group I: Normal control (Perforations was observed), B. Group II: Standard [Omeprazole] (20 mg/kg,bd.wt,p.o). C. Group III: [MTE] (200 mg/kg,bd.wt,p.o), D. Group IV: [MTE] (400 mg/kg,bd.wt,p.o)
Table 5- Effect of Morinda tinctoria extract treatment on Pylorus ligation induced gastric ulcers in rats-
|
Group |
Treatment |
Ulcer index (mean ± SEM) |
pH |
Gastric volume |
Total acidity |
Free acidity |
|
I |
Normal control |
5.82±0.02 |
2.72±0.02 |
1.23±0.03 |
65.1±0.06 |
61.2±0.06 |
|
II |
Standard Omeprazole |
4.53±0.01 |
5.51±0.01 |
0.69±0.01 |
41.03±0.03 |
40.47±0.03 |
|
III |
Received MTE (200mg/kg) |
5.48±0.01* |
3.72±0.04*** |
1.14±0.03* |
61.1±0.06ns |
59.03±0.09ns |
|
IV |
Received MTE (400mg/kg) |
4.92±0.02*** |
4.59±0.01*** |
0.87±0.03*** |
51.03±0.03*** |
41.27±0.03*** |
|
Values are expressed as mean ± SEM, (n = 4). Statistical analysis was performed by using ANOVA by Dunnett's test. Results were compared NC [P < 0.001*] Standard [p < 0.001 ***], [ns = non significant], MTE- Morinda tinctoria extract |
||||||
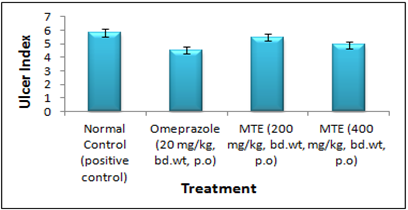
Figure 4- Ulcer Index of the hydro-alcoholic extract of Morinda tinctoria Pylorus ligation induced gastric ulcers in rats
3.2 Swimming Stress-induced gastric ulcers in rats-
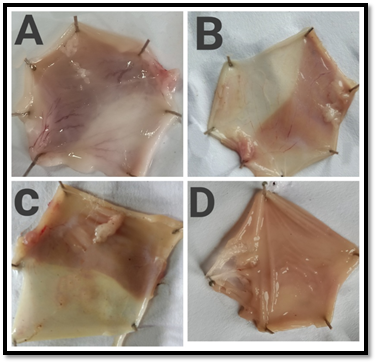
Figure 5- Macroscopic appearance of the gastric mucosa in Swimming stress induced ulcer model- A. Group I: Normal control (Perforations was observed), B. Group II: Standard [Famotidine] (20 mg/kg,bd.wt,p.o). C. Group III: [MTE] (200 mg/kg,bd.wt,p.o), D. Group IV: [MTE] (400 mg/kg,bd.wt,p.o)
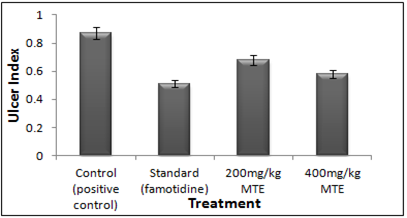
Figure 6- Ulcer Index of the hydro-alcoholic extract of Morinda tinctoria on swimming stress-induced peptic ulcer
3.3 Aspirin-induced gastric ulcers in rats-
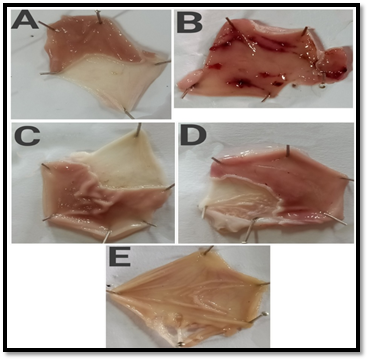
Figure 7- Macroscopic appearance of the gastric mucosa in aspirin-induced ulcer model- A. Group I: Normal control, B. Group II: Disease control (Perforations was observed), C. Group III: Standard [Omeprazole] (20 mg/kg, bd.wt, p.o). D. Group IV: [MTE] (200 mg/kg, bd.wt, p.o), E. Group V: [MTE] (400 mg/kg, bd.wt, p.o)
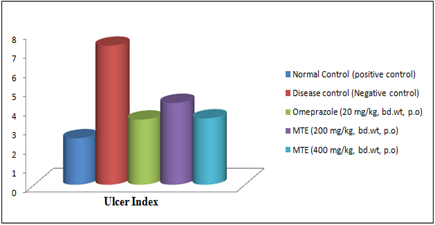
Figure 8- Ulcer Index of the hydro-alcoholic extract of Morinda tinctoria on Aspirin-induced gastric ulcer in rats
3.4 H+-K+ ATPase Inhibition Activity-
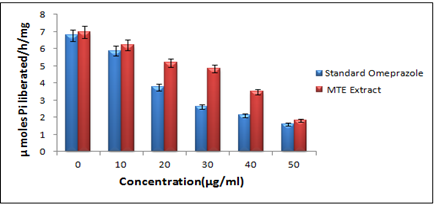
Figure 9- Effect of the hydro-alcoholic extract of Morinda tinctoria and Omeprazole on H+-K+ ATPase activity
Discussion
The pharmacognostic analysis was performed according to the Indian pharmacopeia and total ash was 4.73%, acid-insoluble ash was 0.667%, water-soluble ash was 2.167%, foreign organic matter was 1.8%, loss on drying was 10.34%, alcohol soluble extractive was 56.56%, water-soluble extractive was 7.12%, ether soluble extractive was 13.76% (Table 1). According to the standard procedures, a preliminary phytochemical test of the aerial parts of M.tinctoria was performed, which confirmed the existence of carbohydrates, amino acid, glycosides, flavonoids, alkaloids in the hydro-alcoholic extract (Table 2).
In general, acute and sub-acute toxicity trials are conducted in a few days to two months. The rats treated with the different doses of hydro-alcoholic extract of M. tinctoria (500, 1000, 2000, and 4000 mg/kg) did not affect the weight of the body (Figure 1), and there were no significant differences in the weights of any of the isolated organs between the treated and the control rats. The M. tinctoria did not cause any toxic effect of this measure on the stomach and other organs, as the gross and net weights of the organs were not substantially different from control values (Figure 2). Behavioral changes, therapy-related unusual signs, and death risk did not occur during the mandated study period. No substantial variation was observed in biochemical parameters such as Total Cholesterol, HDL Cholesterol, LDL Cholesterol, Glucose (pre- and post-treatment), and Total Protein. The analysis is presented in (Table 3). No mortality was accounted for in Wistar rats treated at a higher dosage of 4000 mg/kg. All estimated hematological parameters were reported under the healthy levels of rats in the groups being investigated. In both control and treated rats, hematological parameters like RBC, WBC (Total), Hb, and Platelets were not really substantially distinct for MTE. In the extract-treated animals, there were no substantial variations in hematological parameters compared to the control (Table 4), which suggests that there were no lyses of blood cells and/or restriction in the synthesis of blood cells by the active constituents of the MTE. The observations above show that M. tinctoria is non-toxic in rats.
In Pylorus ligation induced gastric ulcer model, animals treated with MTE at dosage levels of 200 as well as 400 mg/kg reported a substantial decrease in total count of ulcers and in the Ulcer Index (UI) (Figure 3). In Aspirin induced ulcer model, 200 mg/kg study group displayed 5.48±0.01 Ulcer Index and some effect relative to the control group, but on comparison with the Omeprazole, it was not statistically significant. On the other terms, the test group of 400 mg/kg revealed 4.92±0.02 (Table 5) Ulcer Index was significantly relevant as contrasted to the control and standard group (Figure 4).
In Swimming Stress-induced gastric ulcers in rats, in 200 mg/kg dose given orally, MTE extract exhibited prevention from ulcer as the ulcer index was 0.68±0.01 and was significant. A reduction in the number of ulcers and the ulcer index was observed (Figure 5). Although taken orally at 400 mg/kg dosage, MTE extract reported 0.58±0.00 and also decreased the incidence of ulcers and the Ulcer Index (UI) significantly. While Famotidine (10mg/kg) was administered, MTE extract revealed a 0.51±0.02 Ulcer Index. Hydro-alcoholic extract of M. tinctoria proclaimed significant lessening in the ulcer index at 400 mg/kg dose comparative to the standard (Figure 6).
In Aspirin-induced gastric ulcers in rats, it was demonstrated that MTE showed considerable protection toward aspirin-induced ulcers at drug concentrations of 200 mg/kg and 400 mg/kg bd. wt. Positive control did not show any ulcer. In negative control (disease control) group in which only aspirin was administered showed a 7.27±0.01 Ulcer Index (UI) with gastric mucosal hyperplasia and inflammation (Figure 7). In 200 mg/kg dose given orally, MTE showed a 4.28±0.011 Ulcer Index (UI). A reduction in the number of ulcers and the ulcer index was observed. Although taken orally at 400 mg/kg dosage, MTE reported 3.47±0.01 Ulcer Index (UI) and no hyperplasia and inflammation were observed. In standard (Omeprazole 20mg/kg) showed a 3.41±0.011 Ulcer index and no inflammation was observed. Significant lessening in the ulcer index at 400 mg/kg dose was observed in comparison to the standard (Figure 8)
In H+-K+ ATPase Inhibition Activity, Dose-dependent enzyme inhibition by Omeprazole and extract was evaluated, indicating that MTE was substantially capable of inhibiting the enzyme H+-K+ ATPase, essential for acid secretion and comparable in action to Omeprazole. The hydro-alcoholic extract of M.tinctoria greatly decreased ATP hydrolysis by goat gastric ATPase with IC50 by 35 μg/mL. As a positive control, Omeprazole (10-50μg/mL) was utilized to decrease the activity of H+-K+ ATPase with IC50= 29 μg/mL (Figure 9)
Conclusion
M. tinctoria extract (MTE) effectively prevents the gastric mucosa from the threat of ulcers caused by pylorus ligation, swimming-stress, and aspirin-induced ulcers. MTE demonstrated a substantial reduction in the ulcer index developed by all 3 experimental models comparable to the standard drugs Omeprazole and Famotidine. In H+-K+ ATPase (in-vitro) inhibition activity, the extract demonstrated substantial proton pump inhibition behavior in the goat gastric mucosal supernatant compared to the standard. Such research results have shown that the hydro-alcoholic extract of MTE is an effective proton pump inhibitor. Current evidence shows that the aerial parts of MTE have substantial antiulcer action.
Acknowledgement
The author wishes to thank Pranveer Singh Institute of Technology, Bhatia-Kanpur, Uttar Pradesh, India, for carrying out practical work and providing literature reviewing support for this study.
Conflicts of interest
The authors declare that there are no conflicts of interest.
References
