The necessity of the development of compounds with effectiveness in therapy against infective diseases has increased in the last 3 years in connection with the spread of COVID-19. The current study aimed to summarize the pharmacological activities of metal-based organic compounds against different viral diseases, including COVID-19. A systematic literature review was carried out by using data sources in Medline, Pub Med, SCOPUS, and Science Direct. The used methods were collecting data, summarizing, and analyzing information. Antiviral properties exert metal complexes with ligands, such as: hydrazones; thiosemi-carbazones: Pt(II), Pd(II); Ga(III); Pd(II); Co(III), Ni(II), Cu(II)); fluoroquinolones, quinoline: Pd(II); phenylquinoline, phenylpyridine; tetrahydropyrimidines: Ag(I); phenanthroline: Cu(II); Valacyclovir: Cu(II)). Complexes of Zn(II). Co(II), Cu(II), Ni(II), Mg(II), Mn(II), and Zn(II) exert antiviral effects against the DNA Herpes simplex viruses HSV-1, HSV-2. Co(II), effective towards the HIV are complexes of the following metals: Au(II), Co(II), Cu(II), Fe(III), La(III), Mg(II). Ni(II), Pd(II), Pt(II), Ru(II). Against the HIV have been described to be active complexes of the following metals: Au(II), Co(II), Cu(II), Fe(III), La(III), Mg(II), Ni(II), Pd(II), Pt(II), and Ru(II). In connection with the continued spread of the severe acute respiratory syndrome – Coronavirus 2 (SARS-CoV-2), today's very actual strategy is the development of effective COVID therapeutics. The perspective trend in the creation and investigation of potential agents for the effective application towards SARS-CoV-2, are Auranofin and Cu(II), Ni(II), Mn(II), and Zn(II) complexes of Coumarin.
Introduction
Metal-Based Organic Complexes as Potential Antiviral Agents
Viral diseases are caused by viruses, which by attachment of their capsid proteins to the receptors of the surface of living cells can penetrate cells through receptor-mediated endocytosis. Virus replication is accomplished through the synthesis of viral messenger RNA (mRNA) and proteins, which multiply the genome. Replication of DNA viruses occurs within the cellular nucleus, whereas RNA virus replication occurs in the cytoplasm.
It has been reported that viruses such as Herpes, Coxsackie B, Influenza A can influence the induction of autoimmune diseases [1].
Different types of viruses affecting humans are classified as follows:
The Baltimore classification of viruses is predicated on the various mRNA production mechanisms. Single-stranded ssRNA or ssDNA, double-stranded dsRNA or dsDNA, and reverse transcriptase (RT) or non-RT-dependent dsRNA or dsDNA are utilized to separate viral genomes. The Baltimore classification includes the following seven categories of viruses:
Viral diseases caused by viruses are summarized in Table 1 [1]:
Table 1. Viral diseases are caused by different virus types.
|
Viral diseases |
Virus types |
|
AIDS (acquired immunodeficiency syndrome) |
HIV (Human immunodeficiency virus) [2] |
|
Chikungunya fever |
Chikungunya virus (Alphavirus) (Togaviridae) [3] |
|
Coronavirus disease 2019 (COVID-19) |
Severe acute respiratory syndrome Coronavirus 2 (SARS-CoV-2) [4] |
|
Dengue viruses (DEN-1, DEN-2, DEN-3 and DEN-4) – Flavi viruses [3, 5] |
|
|
Ebola hemorrhagic fever (Ebola virus disease) |
Ebola virus (EBOV) [6] |
|
Hepatitis A, B, C, D, E diseases |
Hepatitis A, B, C, D, E viruses [7] |
|
Herpes simplex diseases |
Herpes simplex virus 1 and 2 (HSV-1 and HSV-2) [8] |
|
Human papillomavirus infection |
Human papillomavirus (HPV), Papillomaviridae [9] |
|
Human parainfluenza virus infection |
Human parainfluenza viruses (HPIV) [10] |
|
Influenza A/Victoria 3/75, influenza A/Jena 48/78, influenza A/fowl plague, influenza B/Johannesburg) |
Orthomyxoviridae species [11] |
|
Newcastle disease |
Avian paramyxovirus serotype-1 (APMV-1), Paramyxoviridae [12] |
|
Vaccinia |
Orthopoxvirus (Poxviridae) [13] |
|
Zika virus [3] |
Organic metal complexes possess potential activity against different viral diseases [14, 15]. Metal complexes of Curcumin [16], flavonoids [17], chalcones [18] and organic complexes with the following different metals: Ag(I) [19], Co(II) [20], Cu(II), Fe(III), Ni(II), Zn(II), Ti(IV) [21], Co(II), Cu(II), Ni(II), Zn(ii) [22], Pd(II) [23] have been reported as antiviral agents.
Antiviral properties exert metal complexes with ligands, such as:
Complexes of Au, Co, Cu, Fe, Mn, Ni, Pd, Ru, Zn, and V have been described to exhibit potential antiviral properties against different types of viruses [34]. Cytomegalovirus can be blocked by metal complexes of 6-arylthio-3-hydroxypyrimidine-2,4-diones [35]. It has been observed the increased aggregation of Papillomavirus in vitro as a result of the action of Cu(II)-anthracenyl terpyridine complex [36]. It has been shown that ruthenium-p-cymene complexes [37], Pd(II) and Pt(II) complexes with 2-(diphenylphosphino) benzaldehyde 1-adamantoylhydrazone [25]. exert antiviral activity towards poliovirus type 1 [37].
Herpes Simplex
Acylhydrazone-based ligands and their transition metal complexes have been studied as antiviral molecules. Complexes of N'-(2-hydroxy-3-methoxybenzylidene)-2-hydroxybenzoyl hydrazone with Co(II), Cu(II), Ni(II), Mg(II), Mn(II) and Zn(II) possess activity against the DNA Herpes simplex viruses HSV-1, HSV-2 [24].
Pd(II) complexes of pyridine-2-carbaldehyde thiosemicarbazone are active toward HSV-1 [26]. Pd(II) complexes of benzylbis(thiosemicarbazone) and 3,5-diacyl-1,2,4-triazole bis (4-methylthiosemicarbazone) exert effect against the replication of Acyclovir-resistant Herpes simplex virus type 1 and type 2. Throughout the inhibition of the expression of HSV-1 protein gG protein gD, and protein gD, metal complexes suppress the process of the transactivation of the virus genome. The mechanism of action of complexes also is connected with the decreasing of the process of transfer of infection from one the another cell [29].
It has been demonstrated, that cyclometalated iridium(III) complexes, containing chelating ligands, such as fluorinated phenylpyridine, phenylquinoline, or pyridine-2-carboxylate, can interact hydrophobically with the double helix of HSV-1 and HSV-2 viruses, on which mechanism of action is based their antiviral properties towards these viruses [32].
Bovine lactoferrin metal complexes with manganese, ferric, or zinc ions inhibit HSV-1 and HSV-2 replication in vitro [38]. Cobalt complexes with biguanide derivatives exhibit antiviral activity against Herpes virus type 2 [39]. Herpes simplex has been influenced by ruthenium-p-cymene complexes [37, 40] and by bis-cyclopentadienyl metallocene dichlorides of titanium and molybdenum [41]. Mononuclear Cu(II), Fe(III), Ru(III), and Zn(II) complexes of Acyclovir have been investigated towards the BHV-1 (Bovine herpes virus type-1) [42].
Antiviral properties against Herpes simplex virus include Curcumin-Cu(II) complex [43], Caffeic acid, Rosmarinic acid, and Chicoric acid [44]. The Caffeic acid chelates are effective towards HSV-1 and HSV-2. It has been reported that the antiviral activity of the Caffeic acid Fe(III) complex increases 100-fold in comparison with the activity of Caffeic acid [44].
Orthomyxoviruses
The four influenza genera are part of the seven genera in the family Orthomyxoviridae:
Influenza A virus (IAV), genus Alphainfluenzavirus
Influenza B virus (IBV), genus Betainfluenzavirus
Influenza C virus (ICV), genus Gammainfluenzavirus
Influenza D virus (IDV), genus Deltainfluenzavirus
Complexes of Au, Co, Cu, Fe, Mn, Ni, Pd, Ru, Zn, and V have been investigated to exhibit potential antiviral properties towards Influenza viruses by suppression of the process of replication of viral RNA [34]. It has been described that bis-cyclopentadienyl titanium dichloride exert antiviral effect against Orthomyxoviruses (Influenza A/Victoria 3/75, Influenza A/Jena 48/78, Influenza A/fowl plague, Influenza B/Johannesburg), Orthopoxvirus (Vaccinia), Paramyxovirus (Newcastle disease), and Rhabdovirus (Vesicular stomatitis) [41].
Copper complexes exhibit antiviral activity towards S31N M2 mutation of Influenza A virus [45]. It has been observed that Co(III) biguanide derivatives are active against the California influenza virus [39]. Caffeic acid chelates are effective towards Vaccinia virus [44]. Selenium-ruthenium complex is reported to exert activity towards Influenza virus [46].
Chikungunya Virus
As a result of the Chikungunya virus (Alphavirus of the family Togaviridae), chikungunya fever develops. This arthropod-borne virus is transmitted through the bite of a female Aedes sp. mosquito. Aedes aegypti and Aedes albopictus mosquitoes are the primary vectors. The disease is known to begin in 1950 in Africa, followed by 1955 in Tanzania, 1960 in Bangkok, and 1963 - 1973 in India. In 2004 the disease occurred in Kenya and the emergency for faster spread in the world increased. The beginning in Europe is dated from 2007 when the infection was found in Italy [47].
It has been investigated that an antiviral activity against the Chikungunya virus possesses:
It has been described that triphenylphosphine Au(I) derivatives can effectively inactivate the Chikungunya virus [51]. It has been reported that N-heterocyclic carbene metal derivative: Cu(I)-1,3-bis(2,6-diisopropylphenyl)imidazole-2-ylidene complex inhibits 60 % of the virus replication [52].
It has been observed that the cobalt (III)-thiosemicarbazone complex can play an important role as a potential suppressor of the Chikungunya virus by causing up to 80 % inhibition on virus replication [53]. It has been reported that the platinum (II)-Rimantadine metallodrug can be a promising anti-virus agent. The mechanism of protection from the infection is based on the blockage by the complex of viral entry in human cells [54].
Dengue Virus
Dengue virus is a pathogen which worldwide causes infectious mosquito-borne diseases worldwide. For the first time, it occurred in Jakarta in 1968. The virus is distributed in tropical and subtropical areas. Dengue virus contains 4 serotypes as follows: DENV-1–DENV-4. These viruses belong to the family Flaviviridae and originated from the genus Flavivirus. The process of transmission of the virus to humans is caused by Aedes agypty and Aedes albopictus. Till now Dengue vaccine has not been approved. Therefore to decrease the emergency of the infection, is required the development of effective agents, which can reduce the fast spread of viruses. The Dengue virus NS5 protein is found to be an important target for antiviral agents investigation. Cobalt(II)-Morin (2-(2,4-dihydroxyphenyl)-3,5,7-trihydroxychromen-4-one) complex has been investigated to exert antiviral effect, due to suppression of the process of replication of Denga serotype 2 virus [55]. Anti-Dengue serotype 2 virus activity is also proven to possess also Zn(II) complex of the same ligand (2-(2,4-dihydroxyphenyl)-3,5,7-trihydroxychromen-4-one) [56].
The following compounds are anti-Dengue virus agents:
It has been reported that cobalt-protoporphyrin IX and tinprotoporphyrin IX can inactivate the Dengue and Yellow Fever viruses [60].
Ebola Virus
Ebola virus disease is a viral hemorrhagic fever [61]. First cases of the disease were found in 1976, in Sudan and in Congo near the Ebola river, from which is originated the name of the disease [62]. Complexes of Au, Co, Cu, Fe, Mn, Ni, Pd, Ru, Zn, and V have been shown to exert antiviral effects against Ebola virus by inhibiting virus entry into the cells and by blocking replication of RNA [34]. Ebola pseudotyped virus can be inhibited by Caffeic acid metal complexes [44].
Zika Virus
Zika virus is included in the Flaviviridae family and is spread by Aedes mosquitoes. The disease microcephaly, which occurs after infection with Zika virus is associated with the neural disorder. This disease is characterized by brain defects in newborns [63]. An autoimmune disease called Guillain–Barré syndrome also can be caused by Zika virus infection. Copper(II) or cobalt(III) thiosemicarbazones with an effect on Zika virus have been reported [64].
Organic metal complexes with potential antiviral activity against Herpes simplex, Chikungunya, Dengue, and Zika viruses are presented in Table 2.
Table 2. Metal complexes with potential antiviral activity against Herpes simplex, Chikungunya, Dengue, Zika viruses
|
Metal |
Ligand |
|
Ag(I) |
2-mercapto-3,4,5,6-tetra-hydropyrimidine [19] Mafenide [48] |
|
Co(III) |
Acyclovir: Co(II) [33] pyridine-thiosemicarbazone [49] thiosemicarbazones [64] |
|
Cu(II) |
bis(diisopropylsalicylato) (1,10-phenanthroline) [21] fluoroquinolones [30] Curcumin [43] anthracenyl-terpyridine [36] (2,4,5-triphenyl-1H-imidazole) [58] thiosemicarbazones [64] |
|
Ga(II) |
α-(N)-heterocyclic thiosemicarbazones [22] |
|
Ir(III) |
pyridine-2-carboxylate [32] |
|
Pd(II) |
quinolylmethylphosphonate [31] pyridine-2-carbaldehyde thiosemicarbazone [26] bis(thiosemicarbazone [29] 3,5-diacyl-1,2,4-triazole bis(4-methylthiosemicarbazone [29] |
|
Ru(II) |
p-cymene [42, 50] alpha-phellandrene [50] |
Organic complexes of different methals with equal ligands have been described to exert potential antiviral activity against Herpes simplex, Chikungunya, Dengue, and Zika viruses, and some of the methals and ligands are presented in Table 3.
Table 3. Complexes of different methals with equal ligands with potential antiviral activity against Herpes simplex, Chikungunya, Dengue, and Zika viruses
|
Metal |
Ligand |
|
Fe(III) ne Co(III), ne Cu(II), Ni(II) |
thiosemicarbazone [28] |
|
Cu(II), Ni(II), Zn(II) |
chalcones [18] |
|
Cu(II), Fe(III), Zn(II), Ru(III) |
Acyclovir [42] |
|
Co(II), Cu(II), Fe(III), Ni(II), Zn(II), Ti(IV) |
3,5-disubstituted salicylates [21] |
|
Co(II), Cu(II), Ni(II), Mg(II), Mn(II), Zn(II) |
N’-(2-hydroxy-3-methoxybenzylidene)-2-hydroxybenzoyl hydrazone [24] |
Human Immunodeficiency Virus (HIV)
Human immunodeficiency virus (HIV) is a rotavirus. The disease is spread worldwide and millions of people are infected [65]. Reverse transcriptase is a potential target for therapeutics [66].
Complexes of Au, Co, Cu, Fe, Mn, Ni, Pd, Ru, Zn, and V have been described to exhibit potential antiviral properties against HIV, by the mechanism of suppression of the process of replication of RNA [34]. Metal complexes of a sulfonate-N-donor ligand have been investigated for anti-HIV activity [65].
Research has documented the activity of curcumin boron complex [16], polypyridyl ruthenium complexes [67] and complexes of the subsequent metals against HIV: Au(II) in Auranofin [68], Au(III) [69], Cu(II) [66], Co II, Cu(II), Ni(II), Pd(II) [70], Zn(II) [71], Pt(II), Ru(II) [72], vanadium [73], Fe(II), Fe(III), La (III) [74], Mg(II), Zn(II) (Lactoferrin) [75]. Antiviral activity towards HIV has been shown for metal complexes with ligands, such as imidazole [66], bisthiosemicarbazone [69], bicyclams, porphyrins [70], benzofuran [74]. thiosemicarbazones [76]. Platinum(II) and ruthenium(II) complexes [77], triazolyl Ru(II), Os(II), and Ir(III) complexes [78], and thiouracil metal complexes exert potential anti-HIV effect [79] (Table 4).
Table 4. Organic metal complexes with potential anti-HIVactivity
|
Metal |
Ligand |
|
Au(III) |
bis(thiosemicarbazone) [69] |
|
Cu(II) |
imidazole [66] (pyridoxalthiosemisemicarbazone) [76] |
|
Ni(II) |
[bis(citronellathiosemisemicarbazone) [76] |
|
Ru(II), Os(II), and Ir(III) Pd(II) |
triazole [78] |
|
Fe(III), (Ni) |
porphyrins [70] |
Complexes of different methals with equal ligands have been described to exert potential antiviral activity against HIV (Table 5).
Table 5. Complexes of different metals with equal ligands with anti-HIVactivity.
|
Metal Ligand |
|
|
Co(II), Cu(II), Ni(II), Zn(II), Pt(II) |
bicyclam [70] |
|
Cu(II), Fe(III), La(III), Zn(II) |
(5-(1H-benzo[d]imidazol-2-yl)-1H-pyrrol-3-yl)(6-hydroxy-4,7-dimethoxybenzofuran-5-yl)methanone [74] |
|
Co(II), Cu(II), Fe(II), La(III) |
3-(6-hydroxy-4,7-dimethoxybenzofuran-5-carbonyl)-6H-pyrimido [1,6-a]pyrimidine-6,8(7H)-dione [74] |
The FDA-approved gold-containing drug Auranofin for the treatment of rheumatoid arthritis has been investigated for therapeutic application towards cancer, neurodegenerative disorders, and HIV [67, 68]. The potent antiviral effect of bovine lactoferrin, saturated with ferric, manganese, or zinc ions is a result of its mechanism of action, which includes inhibition of the replication of DNA of HIV [57].
The metal bicyclam complexes (JM3100, JM3462, JM3469, JM3461, and JM3158), containing Cu, Co, Ni, Zn, and Pd, respectively, represent a new class of highly effective HIV inhibitors. The important characteristic of these compounds is their selectivity against HIV. The most active is JM3100: 1,1’-[1,4-phenylenebis-(methylene)]-bis-1,4,8,11- tetraazacyclotetradecane octahydrochloride dehydrate. Metalloporphyrins with Fe and Ni have been reported to suppress the process of replication of HIV [70].
COVID‐19
The severe acute respiratory syndrome – Coronavirus 2 (SARS-CoV-2) is the pathogen responsible for the worldwide pandemic COVID-19, which has had an impact on millions of individuals across the globe [80]. The World Health Organization formally declared the outbreak on March 11, 2020 [81]. ACE 2 receptors of alveolar cells are targets of the virus [82]. The disease is characterized by cytokine storm syndrome [83, 84]. SARS‐CoV‐2 replication, transcription, and infectivity depend on polyproteins, which depend on both processes of replication and transcription of SARS‐CoV‐2 virus [81, 82]. The inhibition of the RNA polymerase complex can effectively lead to the suppression of the replication process in viruses [80]. Therefore the inhibition of the RNA polymerase complex is an important target for the mechanism of action and potential antiviral agents.
In accordance with the continued spread of SARS-CoV-2 and increasing deaths [85]. the development of transition metal complexes for antiviral applications is an important trend as the alternative treatment against SARS-CoV-2 [86].
Metal complexes with potential antiviral application against SARS-CoV-2 as therapeutics [41, 85, 87] are:
Auranofin was approved by the FDA in 1985 as an antirheumatic drug. Injectable antiarthritic gold drugs are Disodium aurothiomalate, Aurothioglucose, and Sodium aurothiopropanol sulfonate. Auranofin possesses a potent antimicrobial effect against multidrug-resistant pathogens like Staphylococcus aureus and Enterococcus faecalis. Auranofin exerts antiparasitic action towards the parasite Leishmania infantumq [95, 96]. In comparison Sodium stibogluconate (Pentostam) (Figure 1) is intravenosal anti-Leishmanial drug. In comparison, other Au agents as Chloroquinoline complexes of Au(I) are active against malaria. Other approved metal complexes as antiinfective drugs were introduced in 1910 Salvarsan is the first anti-syphilis arsenium drug, and Benzoxaborole (SCYX-7158) and Melarsoprol, which has been developed for oral treatment of human African trypanosomiasis (Figure 2).
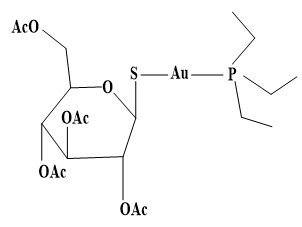 |
|
|
a) |
b) |
|
Figure 1. Structures of Auranofin and Sodium stibogluconate. a) Auranofin. b) Sodium stibogluconate |
|
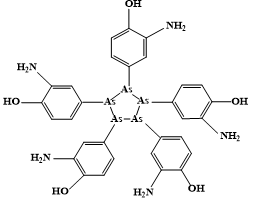 |
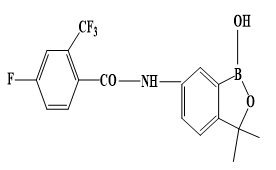 |
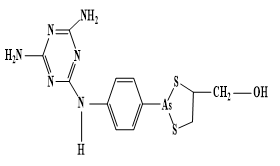 |
|
a) |
b) |
c) |
|
Figure 2. Structures of Salvarsan, Benzoxaborole and Melarsoprol. a) Salvarsan. b) Benzoxaborole. c) Melarsoprol |
||
Auranofin has been proven as a metal-based compound with potential for antiviral application [95]. Auranofin possesses anti-Covid 19 therapeutic activity, based on the ability to inhibit SARS-COV-2 replication by the following mechanisms [90]:
It has been reported that cyclometallated gold(III) complexes [97] and silver N-heterocyclic carbene complexes [98] are potential compounds towards viruses
Conclusion
Organometal complexes are potential antiviral agents against different types of viral diseases in humans as 1) DNA viruses: herpes simplex (HSV-1 and HSV-2), human papillomavirus and human cytomegalovirus; 2) RNA viruses: influenza A, parainfluenza 3, coxsackievirus B3, Chikungunya, Dengue, Ebola, Zika, human immunodeficiency virus. Metal complexes with ligands, such as: hydrazones; thiosemicarbazones: Pt(II), Pd(II); Ga(III); Pd(II); Co(III), Ni(II), Cu(II)); fluoroquinolones, quinoline: Pd(II); phenylquinoline, phenylpyridine; tetrahydropyrimidines: Ag(I); phenanthroline: Cu(II); Valacyclovir: Cu(II)) exhibit antiviral activity. Complexes of Zn(II). Co(II), Cu(II), Ni(II), Mg(II), Mn(II) and Zn(II) possess activity against the DNA Herpes simplex viruses HSV-1, HSV-2. Co(II), An antiviral potential against, Chikungunya, Dengue, Zika viruses possess Cu(II), Ni(II), Mg(II), Mn(II), Zn(II) oranic complexes. Against the HIV have been described to be active complexes of the following metals: Au(II), Co(II), Cu(II), Fe(III), La(III), and Mg(II). Ni(II), Pd(II), Pt(II), Ru(II). In connection with the continued spread of the severe acute respiratory syndrome – Coronavirus 2 (SARS-CoV-2), today's very actual strategy is the development of effective COVID therapeutics. The perspective trend in the creation and investigation of potential agents for the effective application towards SARS-CoV-2, are Auranofin and of Cu(II), Ni(II), Mn(II), Zn(II) complexes of Coumarin, lthiosemicarbazone complexes.
Acknowledgments: None
Conflict of interest: None
Financial support: None
Ethics statement: None
